Question: all are one question but different parts please solve Use the References to access important values if needed for this questioe. According to the following
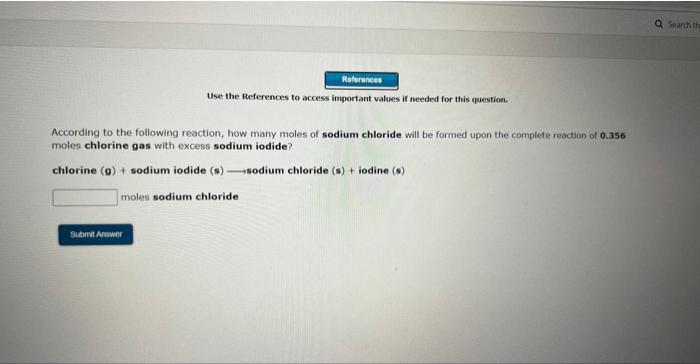
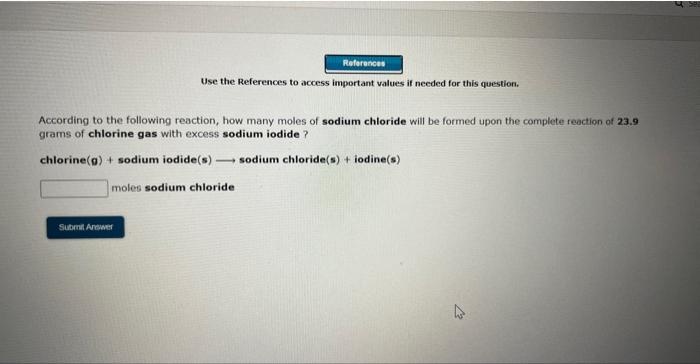
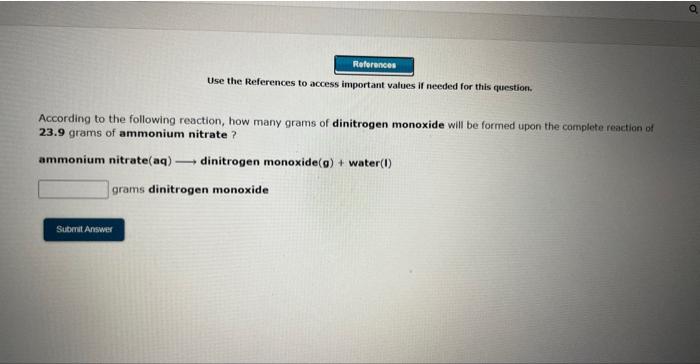
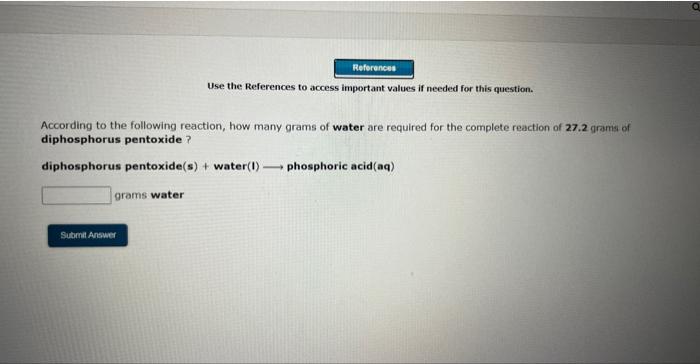
Use the References to access important values if needed for this questioe. According to the following reaction, how many moles of sodium chloride will be formed upon the camplete reaction of 0.356 moles chlorine gas with excess sodium iodide? chlorine (g)+ sodium iodide (s) sodium chloride (s)+ iodine (s) moles sodium chloride Use the References to wccess important values if needed for this question. According to the following reaction, how many moles of sodium chloride will be formed upon the complete reaction of 23.9 grams of chlorine gas with excess sodium iodide ? chlorine (9)+ sodium iodide (s) sodium chloride(s) + iodine(s) moles sodium chloride Use the References to access important values if needed for this question. According to the following reaction, how many grams of dinitrogen monoxide will be formed upon the complete reaction of 23.9 grams of ammonium nitrate ? ammonium nitrate(aq) dinitrogen monoxide(g) + water(I) grams dinitrogen monoxide Use the References to access important values if needed for this question. According to the following reaction, how many grams of water are required for the complete reaction of 27.2 grams of diphosphorus pentoxide? diphosphorus pentoxide(s) +water(I) phosphoric acid(aq) grams water
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


