Question: all please Use the References to access important values if needed for this question. A mixture of nitrogen and neon gases, at a total pressure

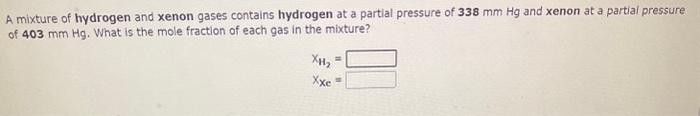

Use the References to access important values if needed for this question. A mixture of nitrogen and neon gases, at a total pressure of 912mmHg, contains 8.07grams of nitrogen and 3.28 grams of neon. What is the partial pressure of each gas in the mixture? PN2=PNe=mmHgmmHg A mixture of hydrogen and xenon gases contains hydrogen at a partial pressure of 338mmHg and xenon at a partial pressure of 403mmHg. What is the mole fraction of each gas in the mixture? Use the References to access important values if needed for this question. A mixture of hydrogen and methane gases at a total pressure of 927mmHg contains hydrogen at a partial pressure of 233mm Hg. If the gas mixture contains 0.145 grams of hydrogen, how many grams of methane are present? Mass=9CH4
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


