Question: Use the References to access important values if needed for this question. The equilibrium constant, Ke, for the following reaction is 0.0290 at 1150K. 2SO3(g)2SO2(g)+O2(g)
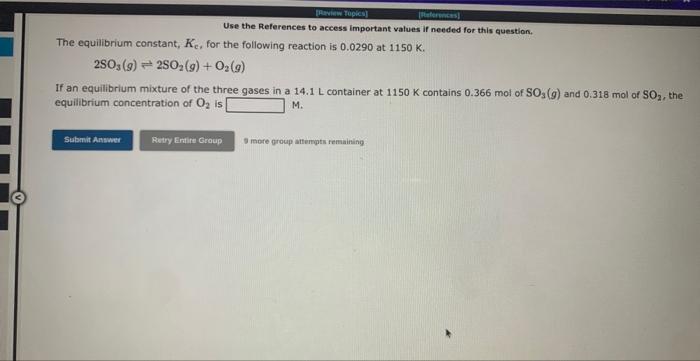
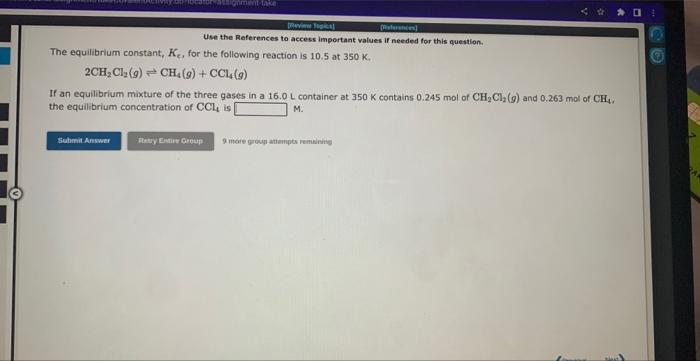
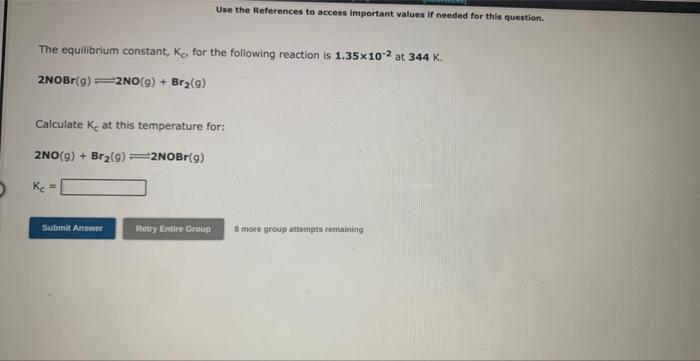
Use the References to access important values if needed for this question. The equilibrium constant, Ke, for the following reaction is 0.0290 at 1150K. 2SO3(g)2SO2(g)+O2(g) If an equilibrium mixture of the three gases in a 14.1L container at 1150K contains 0.366 mol of SO3(g) and 0.318 mol of SO2, the equilibrium concentration of O2 is M. 3 rare group ittempts remaining Use the References to access important values if needed for this question. The equilibrium constant, KE, for the following reaction is 10.5 at 350K. 2CH2Cl2(g)CH4(g)+CCl4(g) If an equilibrium mixture of the three gases in a 16.0L container at 350K contains 0.245mol of CH2Cl2(g) and 0.263mol of CH4. the equilibrium concentration of CCl4 is M. 9 mof greup mtimipts reme ming Use the References to access important values if needed for this question. The equilibrium constant, Kc for the following reaction is 1.35102 at 344K. 2NOBr(g)2NO(g)+Br2(g) Calculate Kc at this temperature for: 2NO(g)+Br2(g)2NOBr(g) Kc=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


