Question: An aqueous solution of a nonionic (that is, nonelectrolyte) solute has a freezing point of 0.362C. The 50 lution contains 2.05g solute dissolved in 94.0gH2O.
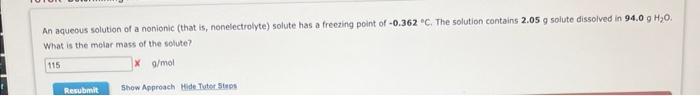
An aqueous solution of a nonionic (that is, nonelectrolyte) solute has a freezing point of 0.362C. The 50 lution contains 2.05g solute dissolved in 94.0gH2O. What is the melar mass of the solute? x a/mal
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


