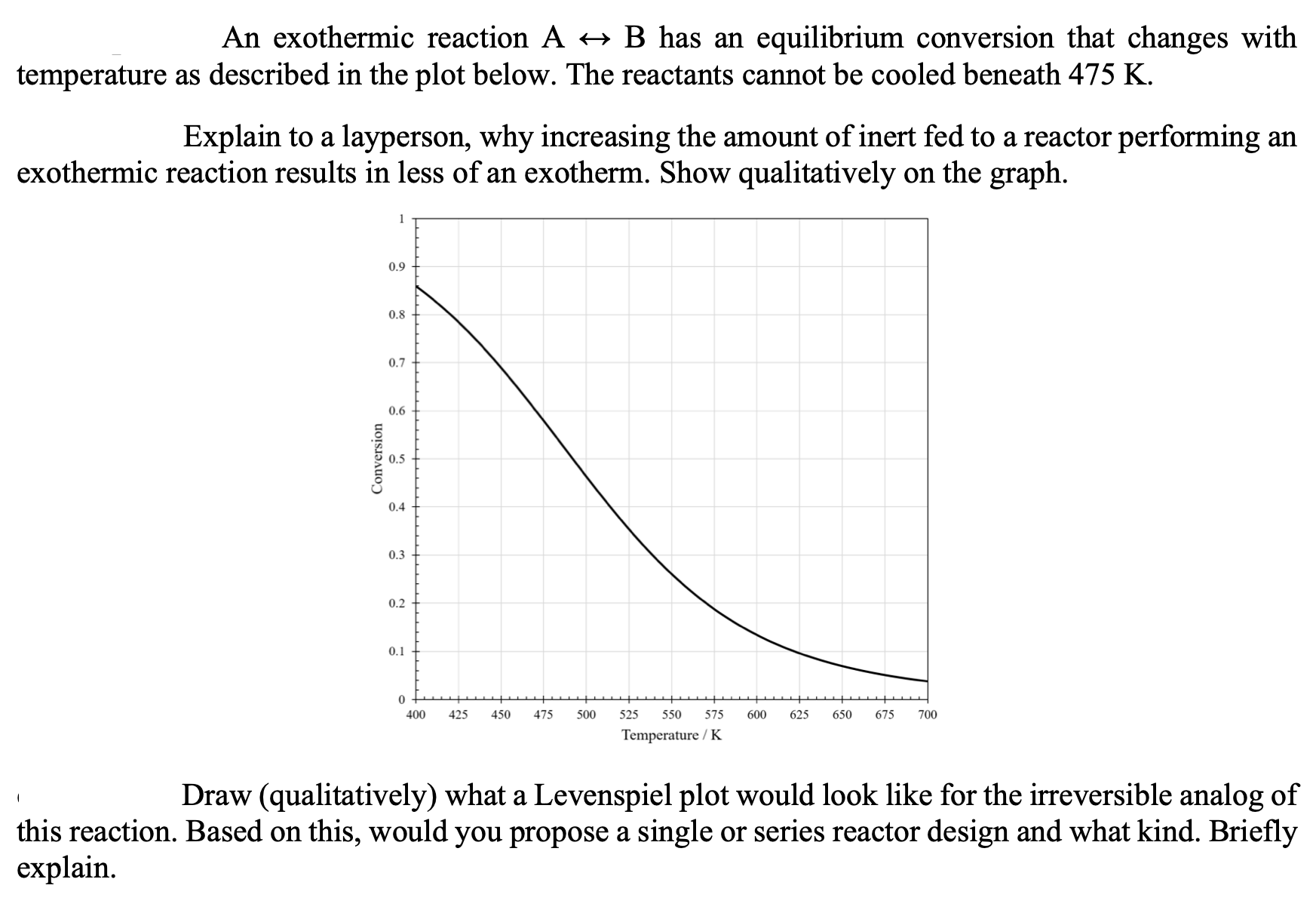
Question: An exothermic reaction A B has an equilibrium conversion that changes with temperature as described in the plot below. The reactants cannot be cooled beneath
An exothermic reaction has an equilibrium conversion that changes with temperature as described in the plot below. The reactants cannot be cooled beneath
Explain to a layperson, why increasing the amount of inert fed to a reactor performing an exothermic reaction results in less of an exotherm. Show qualitatively on the graph.
Draw qualitatively what a Levenspiel plot would look like for the irreversible analog of this reaction. Based on this, would you propose a single or series reactor design and what kind. Briefly explain.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


