Question: And please explain every answer. Thank you 2. The uncharged form of amino acids always occurs between pK1 and pK2. TRUE / FALSE 3. The

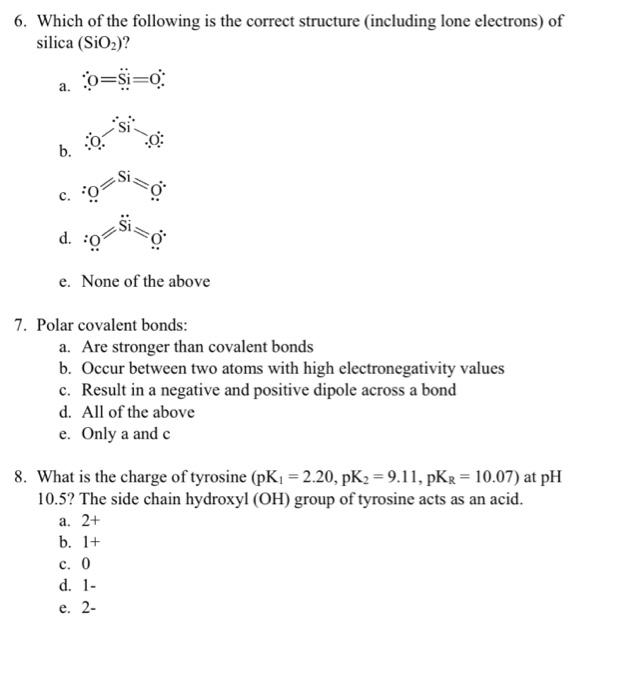
2. The uncharged form of amino acids always occurs between pK1 and pK2. TRUE / FALSE 3. The secondary structure of proteins is determined by between hydrogen bonding between amino acid side chains. TRUE/FALSE PRACTICE Section 2 - Multiple Choice Questions For each question, circle the correct letter. 2 marks for each correct answer. 4. What is the proportion of propionic acid (C2H5COOH;pKa=4.87) in the ionic form (C2H5COO)at pH 5.3 ? a. 17% b. 30% c. 43% d. 73% e. 83% 5. Which of the following is a correct acid base pair? a. H2SO4HSO4 b. HSO4H2SO42 c. SO42HSO4 d. HSO4H2SO4 e. H2SO4SO42 6. Which of the following is the correct structure (including lone electrons) of silica (SiO2) ? a. O=S=O b. c. d. e. None of the above 7. Polar covalent bonds: a. Are stronger than covalent bonds b. Occur between two atoms with high electronegativity values c. Result in a negative and positive dipole across a bond d. All of the above e. Only a and c 8. What is the charge of tyrosine (pK1=2.20,pK2=9.11,pKR=10.07) at pH 10.5? The side chain hydroxyl (OH) group of tyrosine acts as an acid. a. 2+ b. 1+ c. 0 d. 1- e. 2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


