Question: answer and explain 5) A 14.01 g sample of N, reacts with 3.02 g of H; to form ammonia (NH3z). If ammonia is the only
answer and explain
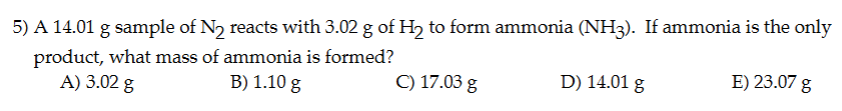
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


