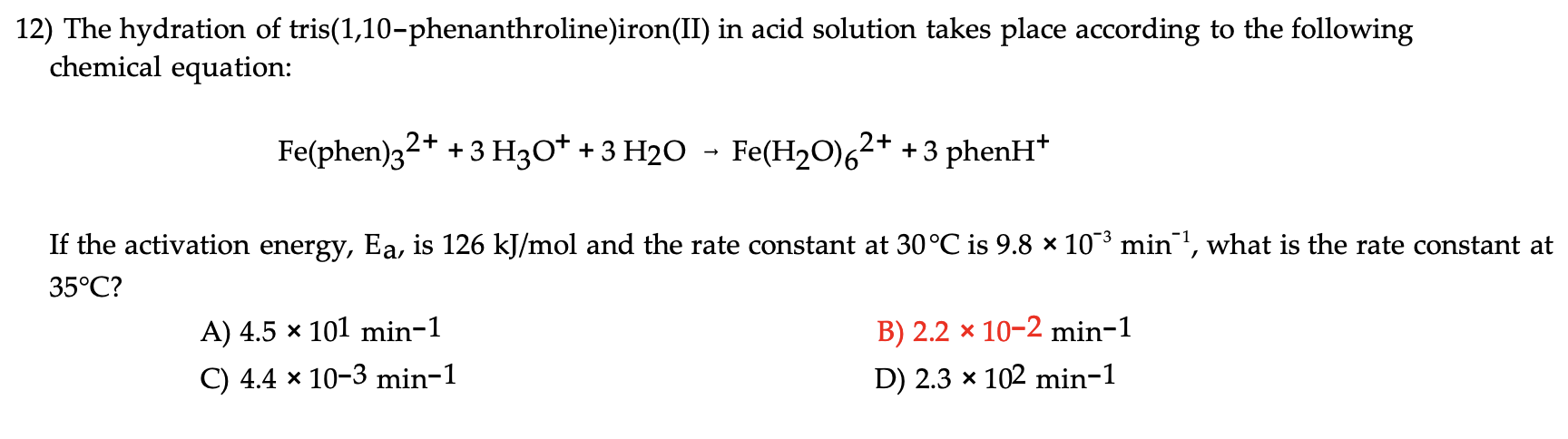
Question: Answer is in red please break it down 12) The hydration of tris(1,10-phenanthroline)iron(II) in acid solution takes place according to the following chemical equation: Fe(phen)32++3H3O++3H2OFe(H2O)62++3phenH+
 Answer is in red please break it down
Answer is in red please break it down
12) The hydration of tris(1,10-phenanthroline)iron(II) in acid solution takes place according to the following chemical equation: Fe(phen)32++3H3O++3H2OFe(H2O)62++3phenH+ If the activation energy, Ea, is 126kJ/mol and the rate constant at 30C is 9.8103min1, what is the rate constant at 35C ? A) 4.5101min1 B) 2.2102min1 C) 4.4103min1 D) 2.3102min1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


