Question: answer key says its E but why? i will upvote! 76. Consider the following oxidation-reduction reaction: + 10,- + H2O MnO4 + 1/2 12 +
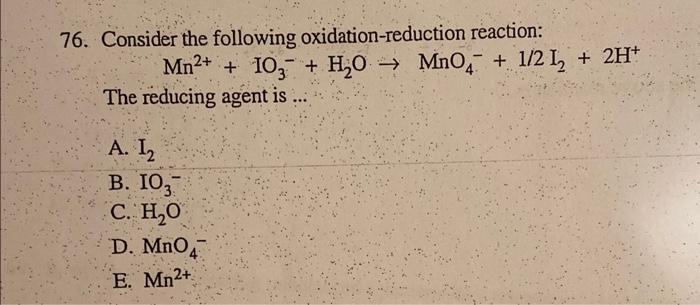
76. Consider the following oxidation-reduction reaction: + 10,- + H2O MnO4 + 1/2 12 + 2H+ The reducing agent is Mn2+ A. IL B. 103 C. H2O D. MnO4 E. Mn2+
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


