Question: Answer Quickly For Thumbs up 2. a) 1500 kg h-1 of a 18% (by mass) solution of isopropyl alcohol in water is being distilled to
Answer Quickly For Thumbs up
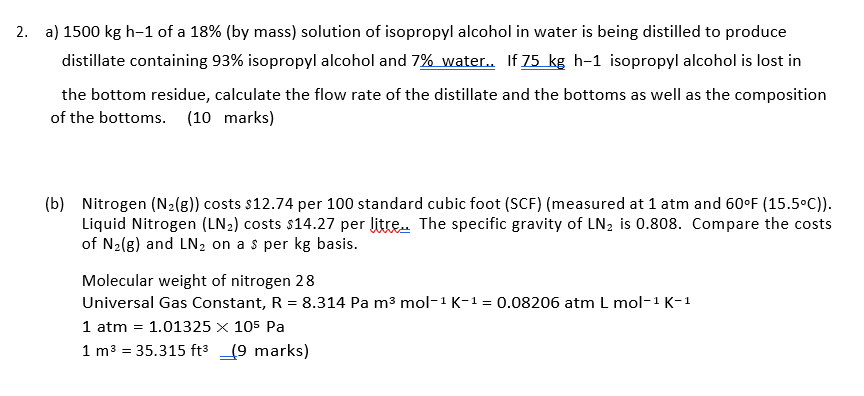
2. a) 1500 kg h-1 of a 18% (by mass) solution of isopropyl alcohol in water is being distilled to produce distillate containing 93% isopropyl alcohol and 7% water. If 75 kg h-1 isopropyl alcohol is lost in the bottom residue, calculate the flow rate of the distillate and the bottoms as well as the composition of the bottoms. (10 marks) (b) Nitrogen (N2(g)) costs $12.74 per 100 standard cubic foot (SCF) (measured at 1 atm and 60F (15.5C)). Liquid Nitrogen (LN2) costs $14.27 per litre. The specific gravity of LN2 is 0.808. Compare the costs of N2(g) and LN2 on a s per kg basis. Molecular weight of nitrogen 28 Universal Gas Constant, R = 8.314 Pa m3 mol-1 K-1 = 0.08206 atm L mol-1 K-1 1 atm = 1.01325 X 105 Pa 1 m3 = 35.315 ft3 _19 marks) =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


