Question: Answers given , answer post lab Q, thank you! Post-Lab Questions 1. Write a net ionic equation for each of the double displacement reactions in







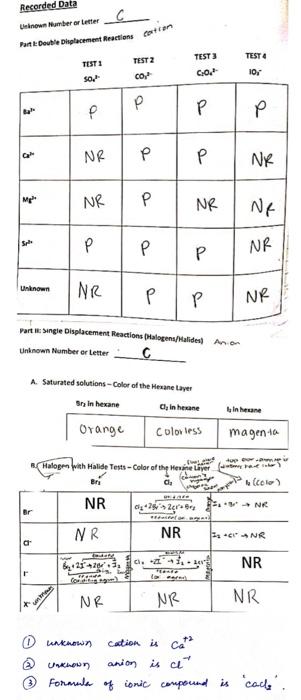
Post-Lab Questions 1. Write a net ionic equation for each of the double displacement reactions in Part I that produced a precipitate. 2. What trend in solubility can you infer for the alkaline earth metals as it relates to position on the periodic table? In other words, do the alkaline earth metal cations become more soluble or less soluble as you move down the family? Most soluble Least soluble 2. What trend in solubility can you infer for the alkaline earth metals as it relates to position on the periodic table? In other words, do the alkaline earth metal cations become more soluble or less soluble as you move down the family? Most soluble Least soluble 3. Write a net ionic equation for each of the single displacement reactions in Part IIb. For those that did not produce a reaction, simply put N.R. (no reaction) for the product. Halogens should be noted as liquids, and halide anions as aqueous substances. 4. Based on the results of the single displacement reactions tested in Part IIB, which halogen (Cl, Br or l) has the greatest oxidizing power? Explain how you know. 5. Based on the results of the single displacement reactions tested in Part IIB, which halogen (Cl, Br or I) has the weakest oxidizing power? Explain how you know. 6. Place the three halogens in order of increasing oxidizing strength. What trend in oxidizing strength can you infer for the halogens as it relates to position on the periodic table? In other words, do the halogens increase or decrease in oxidizing strength as you move down the family? Weakest Strongest Oxidizer 7. Based on the trend you observed, would you expect a reaction to occur between fluorine and your unknown halide? Explain your answer. 8. Do you expect fluorine to be the strongest or weakest oxidizer among the halogen family? Which halide would you react it with to test your theory? Recorded Data Linn Mumber or Letter Partit Double Displacement Reactions or TEST: SO TEST 2 co TEST 3 0.0." TEST 4 10, P P P P P NR P P NK Mp NR P P NR. Ne Sche P NR Unknown NR P NR Partngle Displacement Reactions Halogens/alides in on Unknown Number or Letter C A Saturated solutions - Color of the Heane Layer Sr, in hexane CI, in hexane In hewane Orange Colorless magenta B Halogen With Halide Tests-Color of the Herine Layer ya Bri CLE (color) NR 04+25 26 3. NR Br NR NR 12 CNR a 0. NR NR NR NR 0 unknown Unknown Formular cation is cath anion is ce of ionic compound is calle
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


