Question: arbonate ion, HCO3- is an important natural buffer that allows the maintenance of stable, near-neutral pH ir sh and marine waters. Bicarbonate also controls the
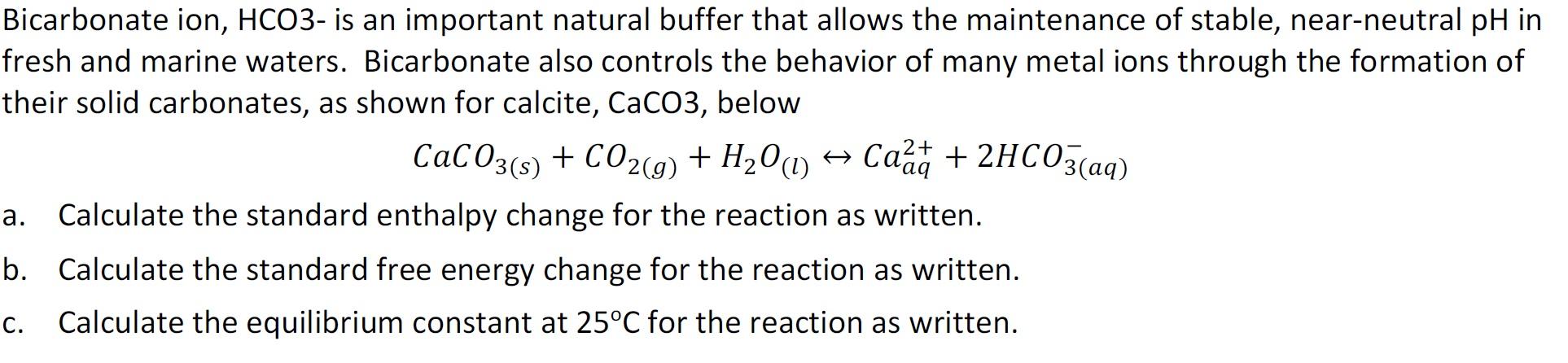
arbonate ion, HCO3- is an important natural buffer that allows the maintenance of stable, near-neutral pH ir sh and marine waters. Bicarbonate also controls the behavior of many metal ions through the formation of eir solid carbonates, as shown for calcite, CaCO, below CaCO3(s)+CO2(g)+H2O(l)Caaq2++2HCO3(aq) Calculate the standard enthalpy change for the reaction as written. Calculate the standard free energy change for the reaction as written. Calculate the equilibrium constant at 25C for the reaction as written
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


