Question: A(s) + 2 B (aq) + C(aq) + Do + E(g) Keq = 4.32 @ 298 K = = If m(A (s))i = 1.7 g,
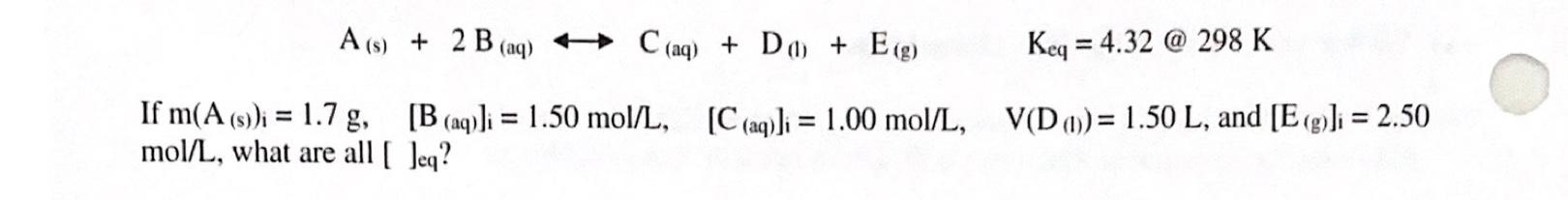
A(s) + 2 B (aq) + C(aq) + Do + E(g) Keq = 4.32 @ 298 K = = If m(A (s))i = 1.7 g, [B (aq)] = 1.50 mol/L, mol/L, what are all (Jeq? [C(aq)]i = 1.00 mol/L, V(D (1))= 1.50 L, and [E (g)]i = 2.50 =
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


