Question: At a certain temperature, the equilibrium constant K for the following reaction is 916.1 H,(8) + 1() -+ 2 HI(g) Use this information to complete
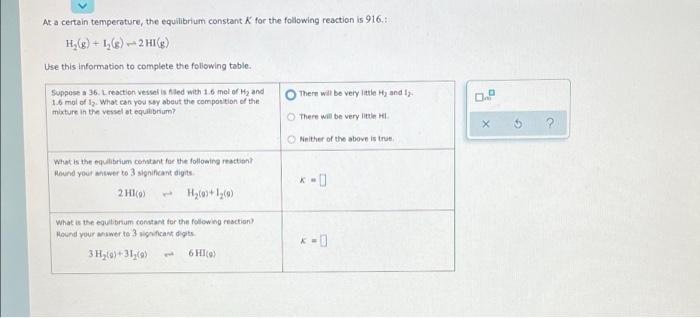
At a certain temperature, the equilibrium constant K for the following reaction is 916.1 H,(8) + 1() -+ 2 HI(g) Use this information to complete the following table. There will be very little Hy and I Suppose a 36. Lreaction vessel is filed with 1.6 mol of Hand 1.6 mol of 1 What can you say about the composition of the mixture in the vessel at equilibrium? There will be very little X 5 ? Neither of the above is true What is the equilibrium constant for the following reaction Round your answer to 3 significant digits 2 H10) H2+1,C) What is the equilibrium constant for the following reaction Hound your swerte 3 fant digits 3H2(0)+31(0) 6 HIO
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


