Question: At a certain temperature, the equilibrium constant K for the following reaction is 4.421010 H2g+I2g 2HIg Use this information to complete the following table. At
At a certain temperature, the equilibrium constant K for the following reaction is 4.421010 H2g+I2g 2HIg 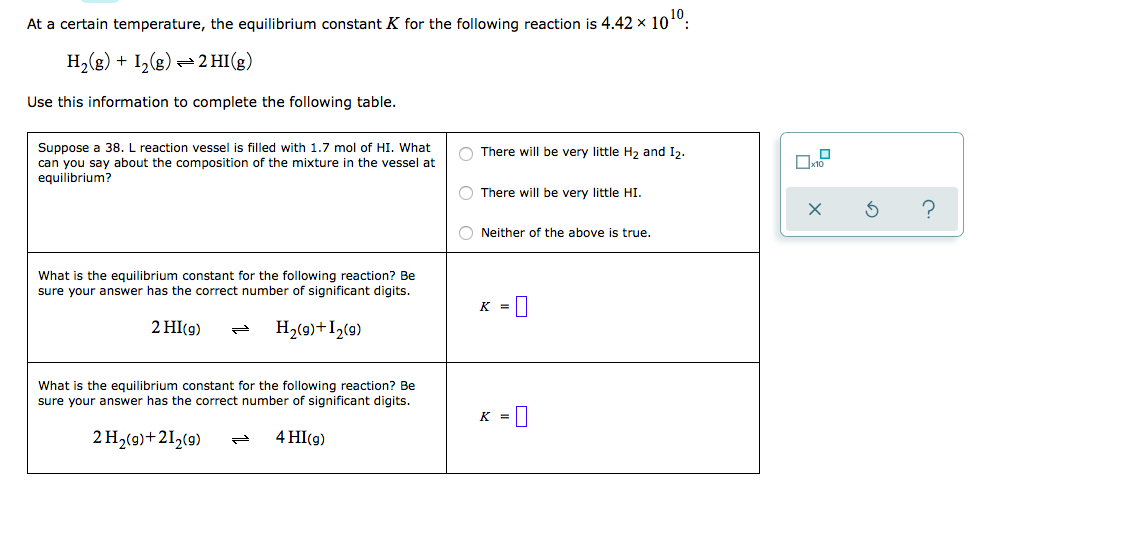 Use this information to complete the following table.
Use this information to complete the following table.
At a certain temperature, the equilibrium constant K for the following reaction is 4.42 x 100: H2(g) + 12(8) = 2 HI(g) + Use this information to complete the following table. There will be very little H2 and 12. Suppose a 38. L reaction vessel is filled with 1.7 mol of HI. What can you say about the composition of the mixture in the vessel at equilibrium? There will be very little HI. 5 Neither of the above is true. What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. K = 0 2 HI(9) H2(9)+12(9) What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. K = 0 2 H2(9)+212(9) 4 HI(g)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


