Question: At room temperature the irreversible second order liquid phase reaction proceeds as follows: A batch reactor takes 18 minutes to fill and empty. What percentage
At room temperature the irreversible second order liquid phase reaction proceeds as follows:
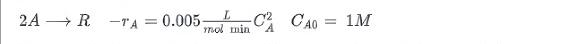 A batch reactor takes 18 minutes to fill and empty. What percentage conversion and reaction time should be used to maximize the daily product output R?
A batch reactor takes 18 minutes to fill and empty. What percentage conversion and reaction time should be used to maximize the daily product output R?
2A R L -7A = 0.005 mol min C3 C40 = 1M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


