Question: Average formation constant (KG) = a) Using the table of solutions below, calculate the initial concentration of Fe+3, SCN, and FeSCN +2 for each solution.
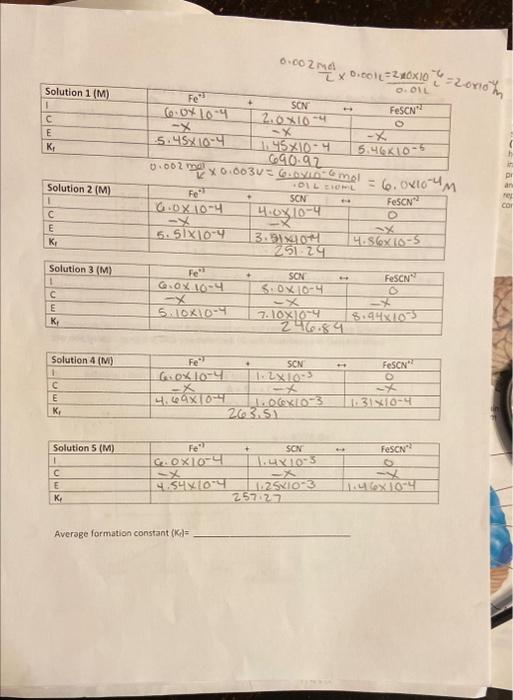
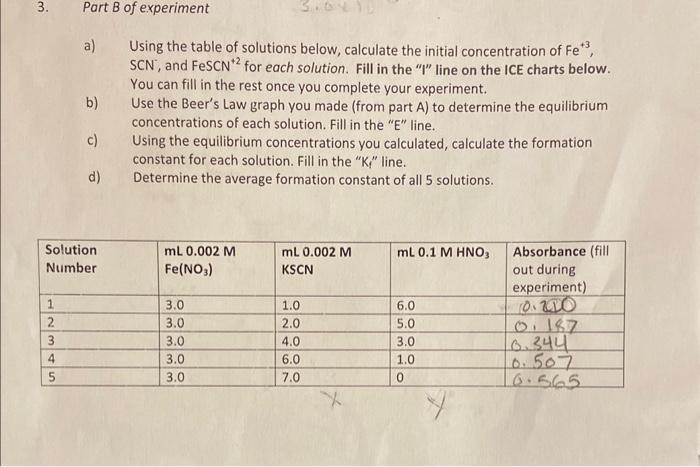

Average formation constant (KG) = a) Using the table of solutions below, calculate the initial concentration of Fe+3, SCN, and FeSCN +2 for each solution. Fill in the "I" line on the ICE charts below. You can fill in the rest once you complete your experiment. b) Use the Beer's Law graph you made (from part A) to determine the equilibrium concentrations of each solution. Fill in the " E " line. c) Using the equilibrium concentrations you calculated, calculate the formation constant for each solution. Fill in the " K " " line. d) Determine the average formation constant of all 5 solutions. Part B Calculations Solution \# 15 Average formation constant (KG) = a) Using the table of solutions below, calculate the initial concentration of Fe+3, SCN, and FeSCN +2 for each solution. Fill in the "I" line on the ICE charts below. You can fill in the rest once you complete your experiment. b) Use the Beer's Law graph you made (from part A) to determine the equilibrium concentrations of each solution. Fill in the " E " line. c) Using the equilibrium concentrations you calculated, calculate the formation constant for each solution. Fill in the " K " " line. d) Determine the average formation constant of all 5 solutions. Part B Calculations Solution \# 15
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


