Question: ( b ) An industrial solvent at boiling conditions ( 2 7 4 K and 1 atm ) has the following effect of pressure on
b An industrial solvent at boiling conditions and atm has the following effect of pressure on temperature at saturated conditions.
The vapor phase and liquid phase volumes are given as: and Calculate the latent heat of vaporization of the solvent.
marks
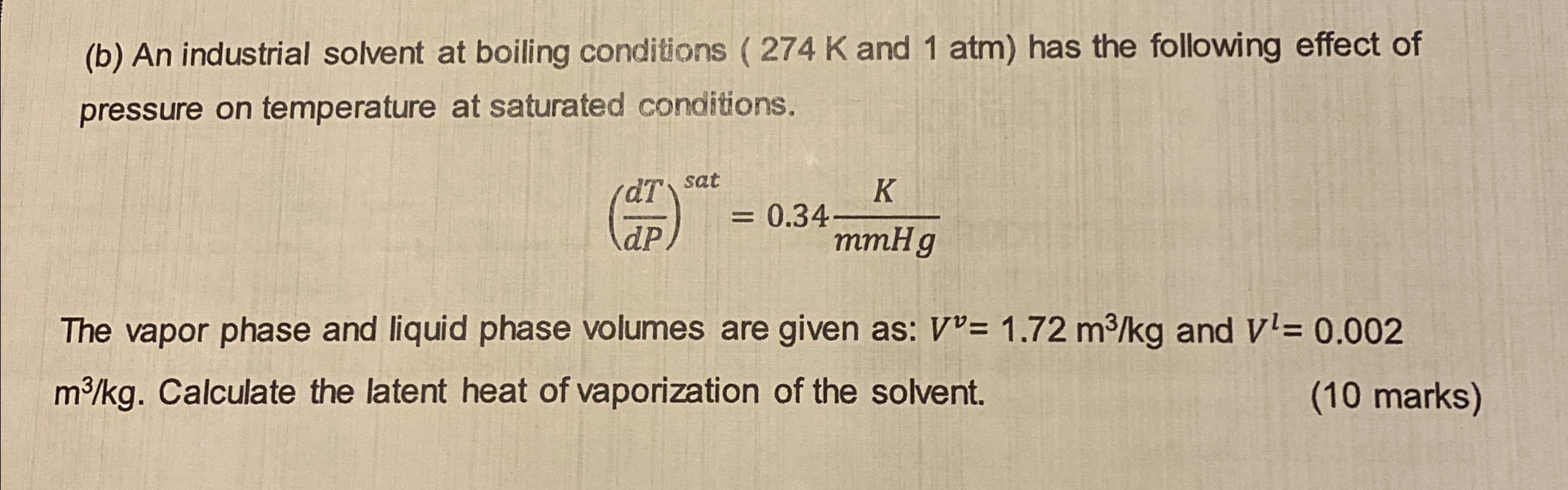
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


