Question: (b) If you start with 0,045Ml2 at this temperature, how much will remain after 5.25 s assuming that the loolite atoms do not recainbine ta
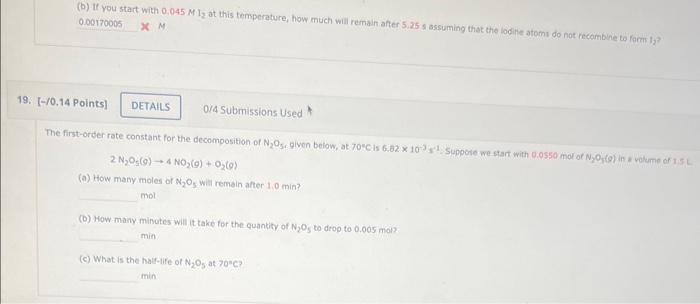
(b) If you start with 0,045Ml2 at this temperature, how much will remain after 5.25 s assuming that the loolite atoms do not recainbine ta farm la? X M. 0/4 Submissions Used 2N2O5(g)4NO2(g)+O2(g) (a) How many moles of N2O5 will remain after 1,0 min? mol. (b) How many minutes will it take for the quantity of N2O5 to drop to 0.005 mol? min (c) What is the half-life of N2O5 at 70C ? min
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


