Question: Balance the following redox reaction in basic solution. Use the method of first assuming the solution is acidic, then add hydroxide ions to counteract added
Balance the following redox reaction in basic solution. Use the method of first assuming the solution is acidic, then add hydroxide ions to counteract added hydrogen ionsIt you complete and balance the following oxidationreduction reaction in basic solution
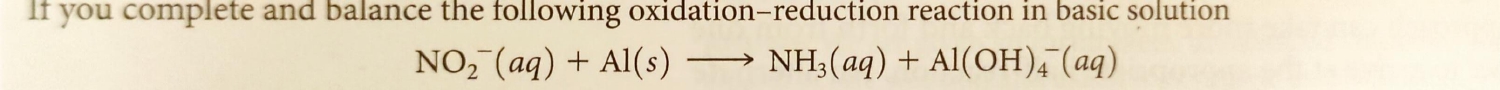
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


