Question: Based on the experimental data, determine the reactor volume RCTA and RFP for afirst order reaction in the gas phase, if T = 2 2
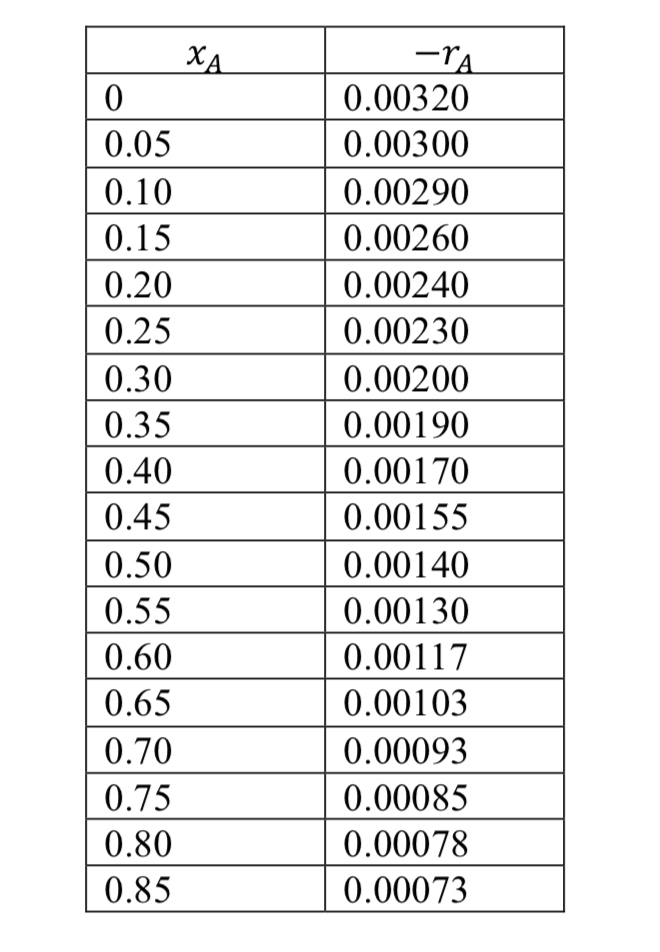
Based on the experimental data, determine the reactor volume RCTA and RFP for afirst order reaction in the gas phase, if T deg C and P atm. Consider flowvolumetric of ls and initial reagent concentration of moldm Looking for a conversion Find the characteristic polynomial for the reaction rate Calculate the volume of the reactor considering that it is an RCTA Calculate the volume of the reactor considering that it is an RFP Draw the corresponding Levenspiel graph, indicating the areas equivalent to eachreactor.table
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


