Question: Be sure to answer all parts. A solution made by dissolving 25mg of insulin in 5.0mL of water has an osmotic pressur mmHg at 25C.
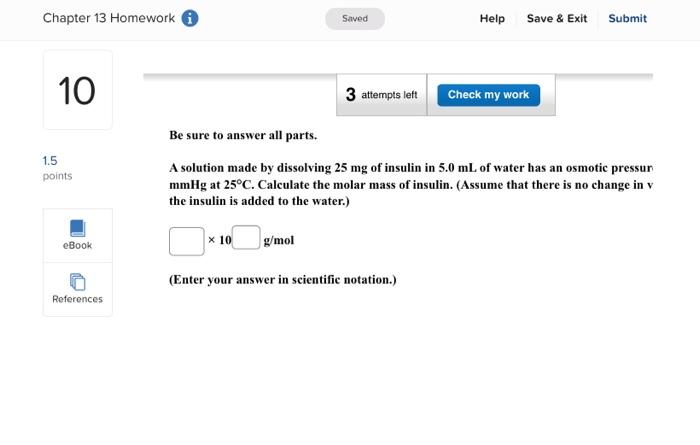
Be sure to answer all parts. A solution made by dissolving 25mg of insulin in 5.0mL of water has an osmotic pressur mmHg at 25C. Calculate the molar mass of insulin. (Assume that there is no change in v the insulin is added to the water.) 10g/mol (Enter your answer in scientific notation.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


