Question: Be sure to answer all parts. Using the balanced equation for the combustion of acetylene, answer the following questions. 2 H-C=CH+502 +4CO2 + 2 H20
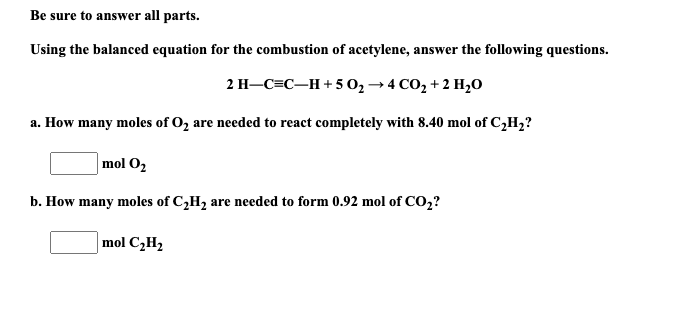
Be sure to answer all parts. Using the balanced equation for the combustion of acetylene, answer the following questions. 2 H-C=CH+502 +4CO2 + 2 H20 a. How many moles of O2 are needed to react completely with 8.40 mol of C2H2? mol O2 b. How many moles of C,H, are needed to form 0.92 mol of CO2? mol C2H2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


