Question: Between these two compounds, NH or CH3COO, draw the Lewis structure of the stronger base. pK's of organic and inorganic acids. Acid Acid CH3
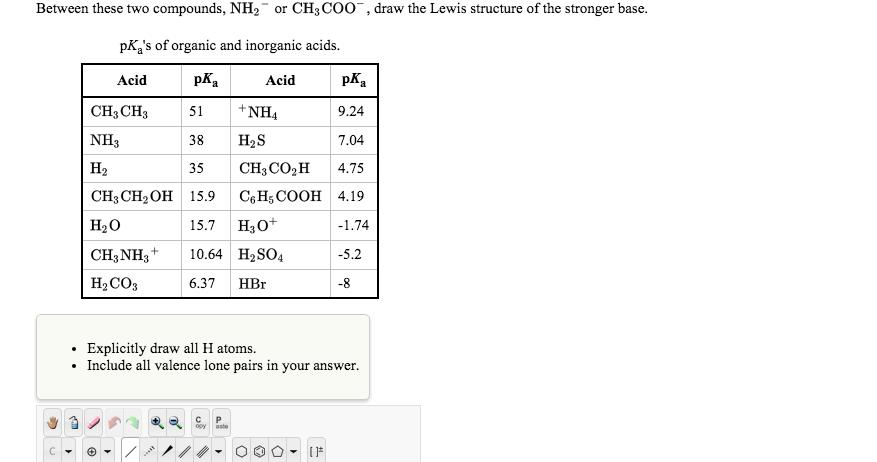
Between these two compounds, NH or CH3COO, draw the Lewis structure of the stronger base. pK's of organic and inorganic acids. Acid Acid CH3 CH3 NH3 H 51 38 35 CH3CHOH 15.9 HO CH3NH3+ HCO3 + pK **** pK 9.24 7.04 CH,COH 4.75 C6H5COOH 4.19 -1.74 +NH HS 15.7 H3O+ 10.64 HSO4 6.37 HBr aste Explicitly draw all H atoms. Include all valence lone pairs in your answer. -5.2 -8 []
Step by Step Solution
★★★★★
3.49 Rating (166 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

The relation between pK value and acidity is as the pK is decreases aci... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


