Question: ble reaction A + B R + S is carried out with the B in the reaction mixture (CAo = CBo = 0.052 ne reaction
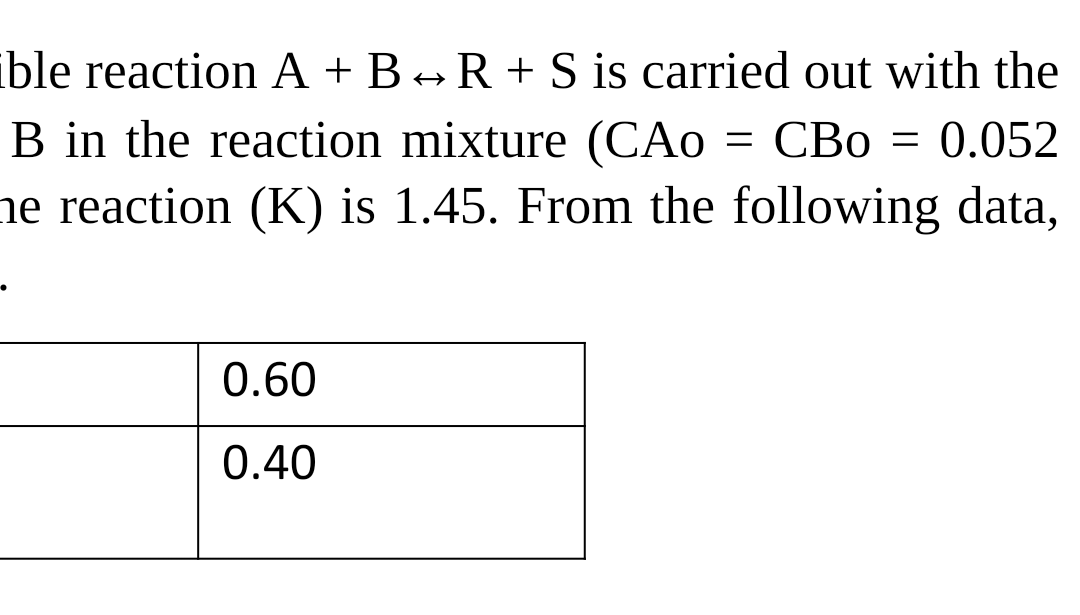
ble reaction A + B R + S is carried out with the B in the reaction mixture (CAo = CBo = 0.052 ne reaction (K) is 1.45. From the following data, - - 0.60 0.40 ble reaction A + B R + S is carried out with the B in the reaction mixture (CAo = CBo = 0.052 ne reaction (K) is 1.45. From the following data, - - 0.60 0.40
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


