Question: Boiling Point Elevation/Freezing Point Depression T=mK where, for freezing point depression: T=T(puresolvent)T(solution) and for boiling point elevation: T=T(solution)T(puresolvent) m= (# moles solute /Kg solvent) Kb=
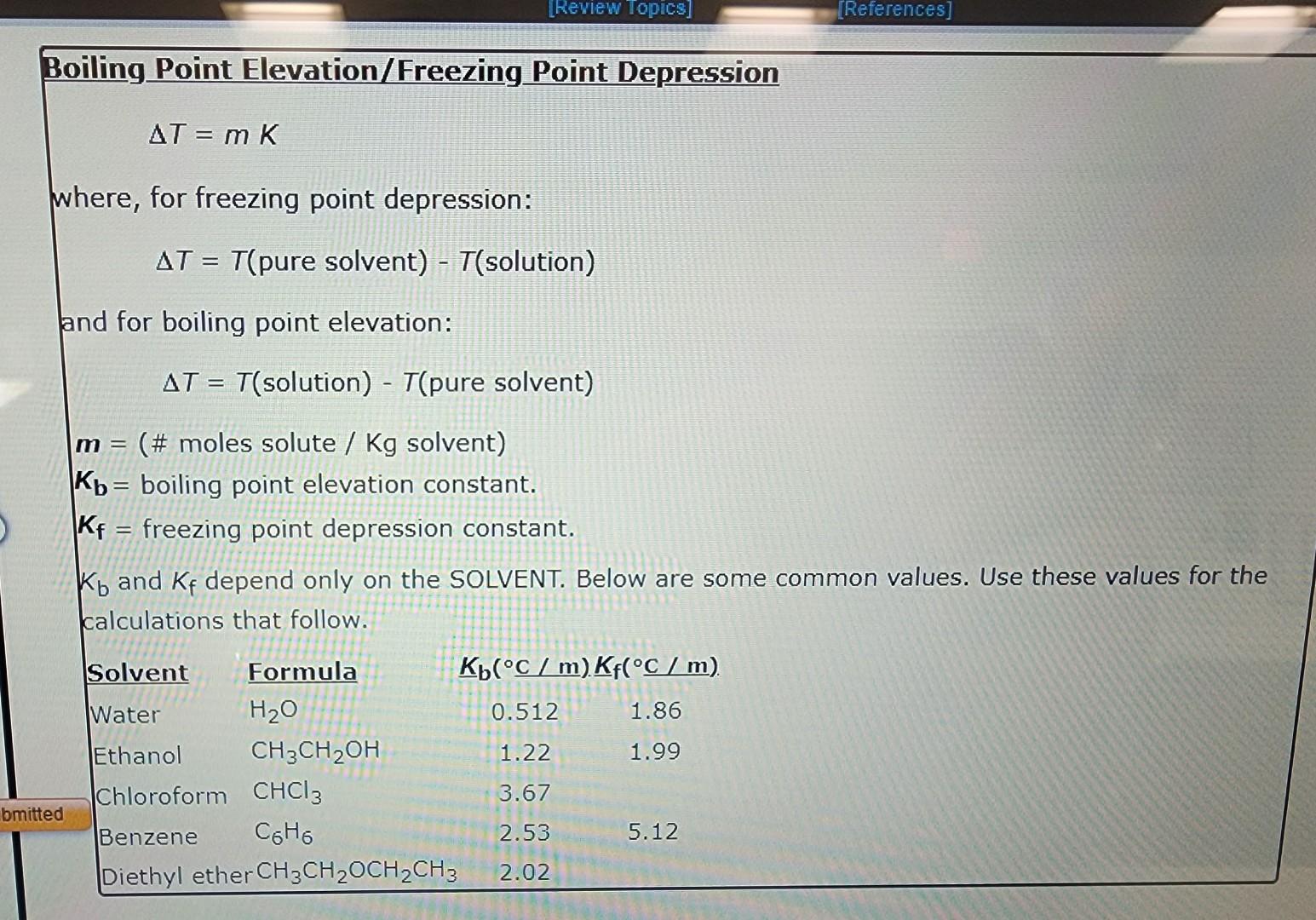
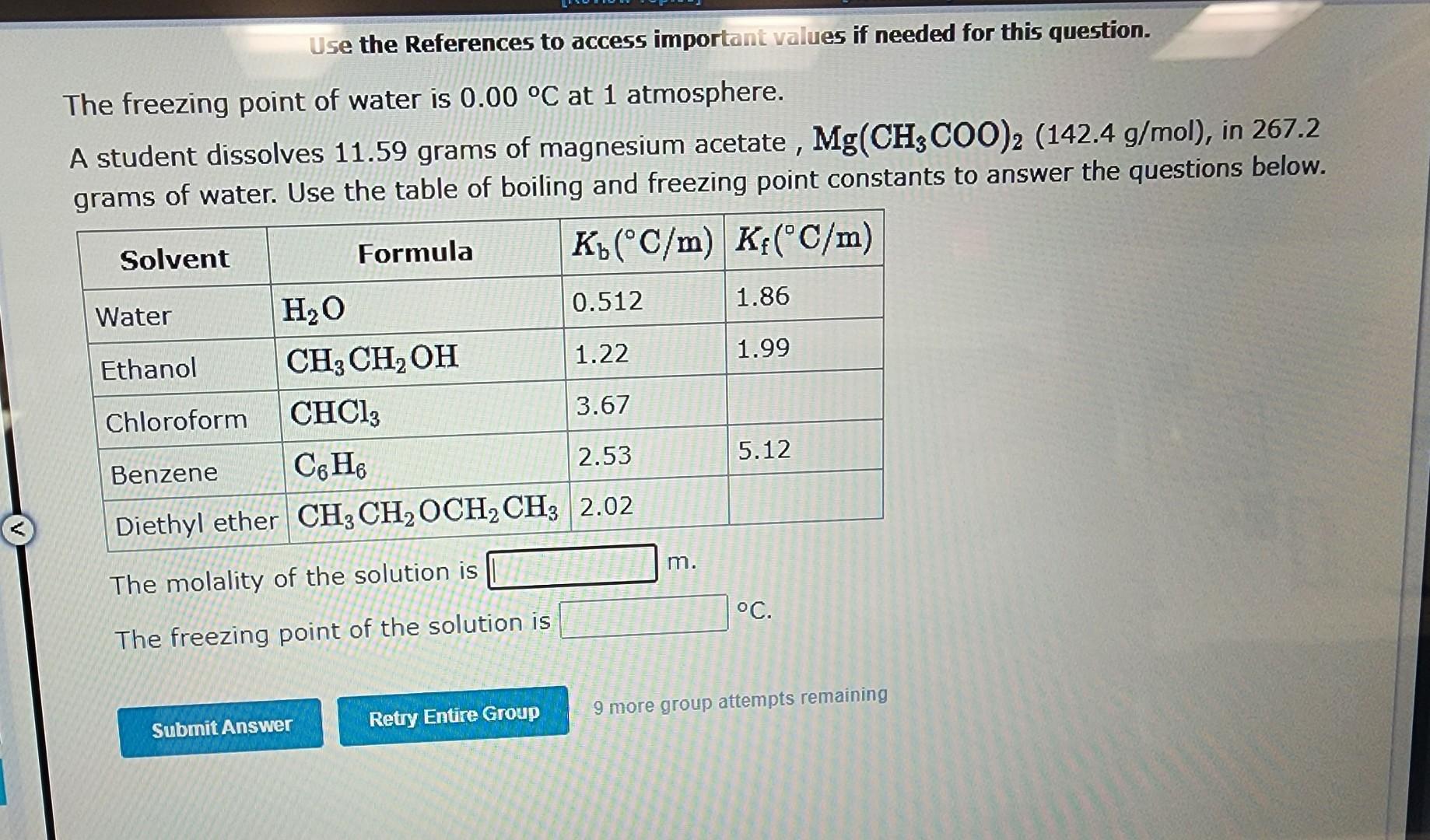
Boiling Point Elevation/Freezing Point Depression T=mK where, for freezing point depression: T=T(puresolvent)T(solution) and for boiling point elevation: T=T(solution)T(puresolvent) m= (\# moles solute /Kg solvent) Kb= boiling point elevation constant. Kf= freezing point depression constant. Kb and Kf depend only on the SOLVENT. Below are some common values. Use these values for the calculations that follow. Use the References to access important values if needed for this question. The freezing point of water is 0.00C at 1 atmosphere. A student dissolves 11.59 grams of magnesium acetate, Mg(CH3COO)2(142.4g/mol), in 267.2 grams of water. Use the table of boiling and freezing point constants to answer the questions below. The molality of the solution is m. The freezing point of the solution is C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


