Question: Both questions please!!! Identify whether each species functions as a Bronsted-Lowry acid or a Bronsted-Lowry base in this net ionic equation. In this reaction: The
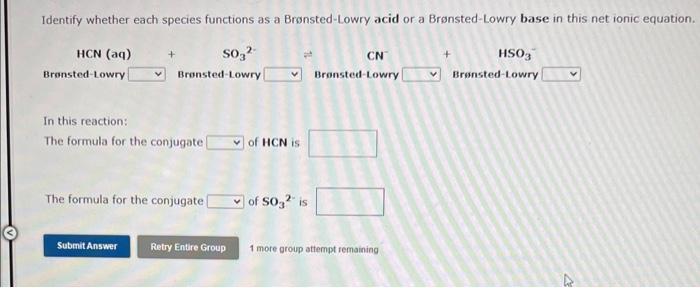
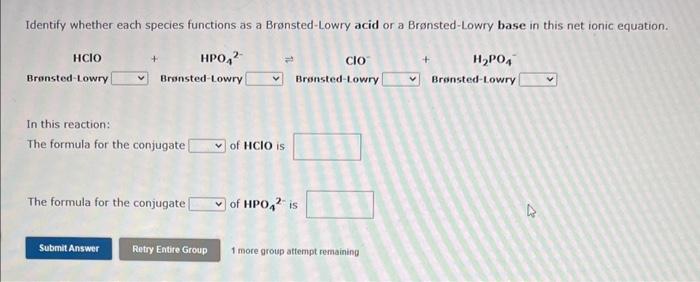
Identify whether each species functions as a Bronsted-Lowry acid or a Bronsted-Lowry base in this net ionic equation. In this reaction: The formula for the conjugate of HCN is The formula for the conjugate of SO32 is 1 more group attempt remaining Identify whether each species functions as a Bronsted-Lowry acid or a Bronsted-Lowry base in this net ionic equation. In this reaction: The formula for the conjugate HClO is The formula for the conjugate of HPO42 - is 1 more group attempt remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


