Question: please help with all THREE questions, thenk you Identify whether each species functions as a Bronsted-Lowry acid or a Brnsted-Lowry base in this net ionic
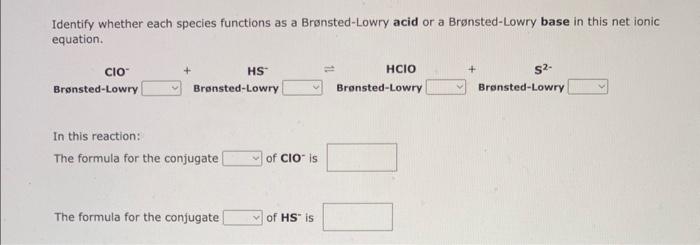
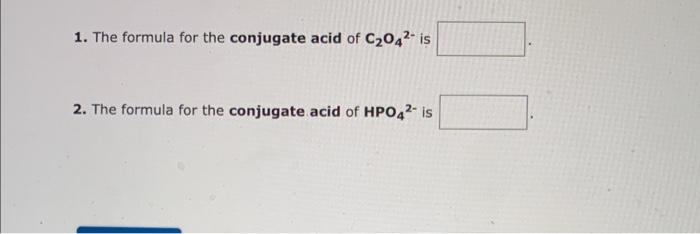
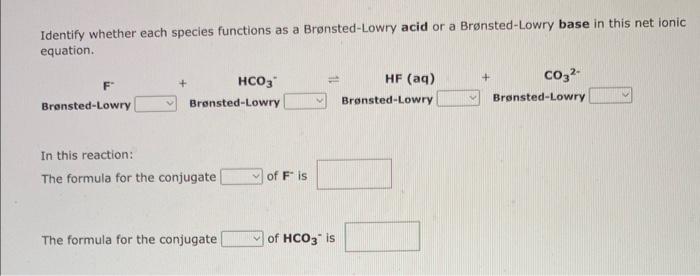
Identify whether each species functions as a Bronsted-Lowry acid or a Brnsted-Lowry base in this net ionic equation. In this reaction: The formula for the conjugate of ClOis The formula for the conjugate of HSis 1. The formula for the conjugate acid of C2O42 is 2. The formula for the conjugate acid of HPO42 is Identify whether each species functions as a Bronsted-Lowry acid or a Brnsted-Lowry base in this net ionic equation. F+HCO3HF(aq)+CO32 In this reaction: The formula for the conjugate Fis The formula for the conjugate of HCO3 - is
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


