Question: plis answer all this is my last question :) i will give you thumbs up :) Identify whether each species functions as a Bronsted-Lowry acid
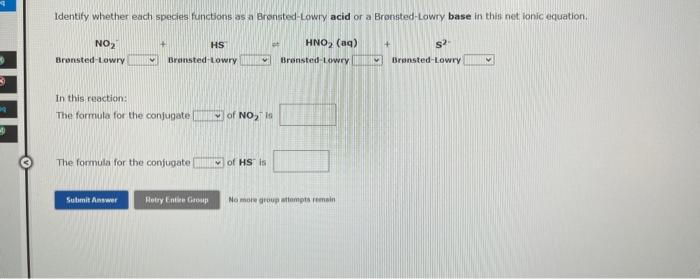
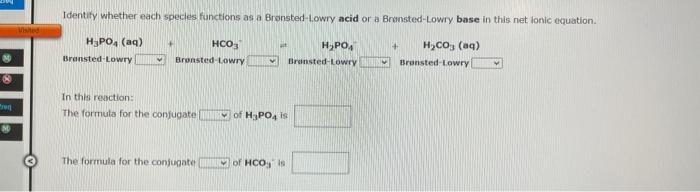

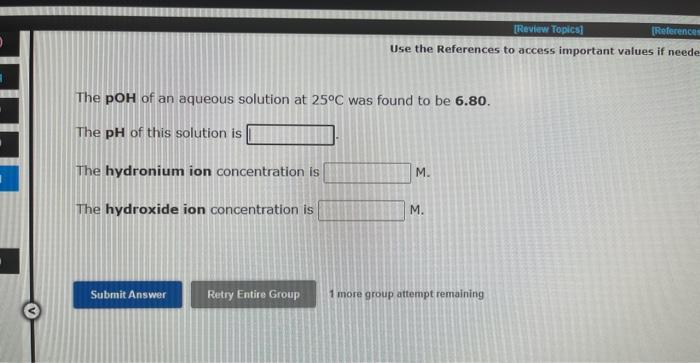
Identify whether each species functions as a Bronsted-Lowry acid or a Bronsted-Lowry base in this net ionic equation. In this reaction: The formula for the conjugate of NO218 The formula for the confugate of HS is No more druup attampts remein Identity whether each specles functions as a Bransted-towry acid or a Brwnsted-Lowry base in this net lonic equation. In the resction: The formula for the confugate of H3PO4 is The formula for the conjugate of HCO3 te The hydroxide ion concentration in an aqueous solution at 25C is 0.045M. The hydronium lon concentration is M. The pH of this solution is The pOH is Use the References to access important values if neede The pOH of an aqueous solution at 25C was found to be 6.80. The pH of this solution is The hydronium ion concentration is M. The hydroxide ion concentration is M. 1 more group attempt remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


