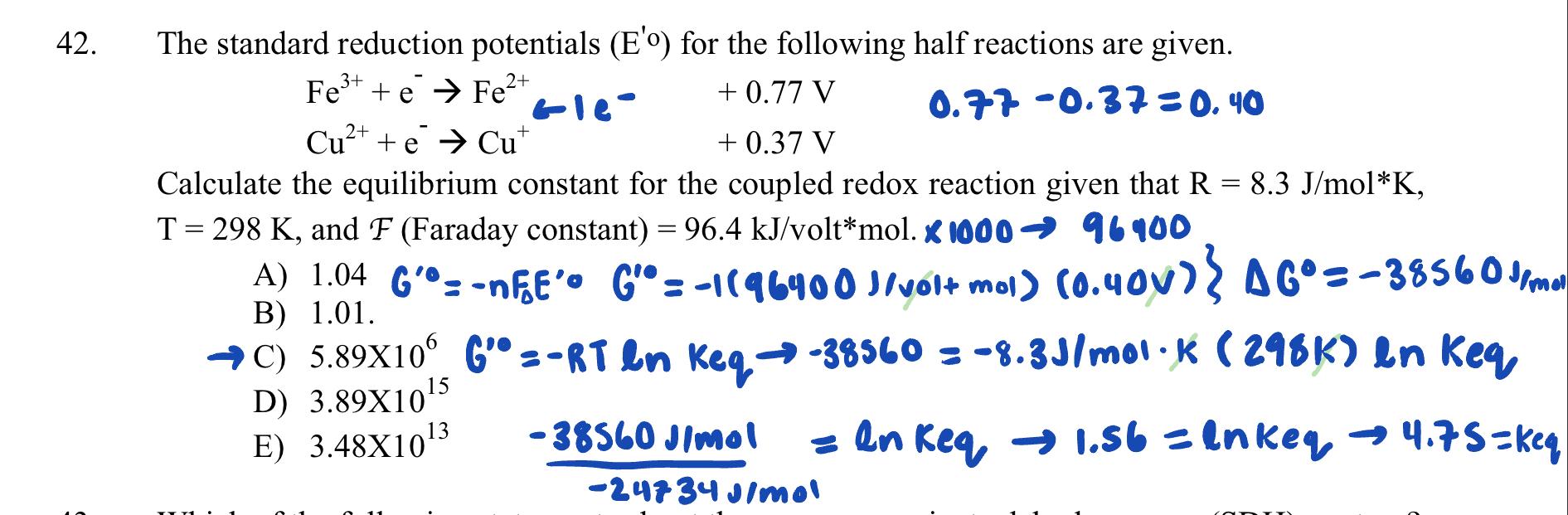
Question: C is the right answer and I don't know what I'm doing wrong. Can someone help? 42. The standard reduction potentials (Eo) for the following
 C is the right answer and I don't know what I'm doing wrong. Can someone help?
C is the right answer and I don't know what I'm doing wrong. Can someone help?
42. The standard reduction potentials (Eo) for the following half reactions are given. Fe3++eFe2+1eCu2++eCu++0.77V+0.37V0.770.37=0.40 Calculate the equilibrium constant for the coupled redox reaction given that R=8.3J/molK, T=298K, and F (Faraday constant) =96.4kJ/ volt*mol. 100096900 C) 5.89106 G 0=RTlnKeq38560=8.3J/molK(298K)lnKeq D) 3.891015 E) 3.4810132483401mol38560Jlmol=lnkeq1.56=lnkeq4.75=ke
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


