Question: Calculate the absorption coefficient at the max values for each of fluorescein, NADH, fully reduced and fully oxidized cytochrome c. Use a pathlength of 1.00cm
Calculate the absorption coefficient at the 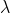 max values for each of fluorescein, NADH, fully reduced and fully oxidized cytochrome c. Use a pathlength of 1.00cm for these calculations. (show the step of calculation) a). fluorescein: use 200
max values for each of fluorescein, NADH, fully reduced and fully oxidized cytochrome c. Use a pathlength of 1.00cm for these calculations. (show the step of calculation) a). fluorescein: use 200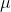 L of 90
L of 90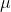 M fluorescein with 800
M fluorescein with 800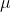 L of 40mM phosphate buffer(pH=7). Since
L of 40mM phosphate buffer(pH=7). Since 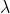 = 490nm has an absorbance 0.87
= 490nm has an absorbance 0.87
b). NADH: 0.250mM NADH.Since 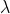 =340nm has an absorbance 1.361
=340nm has an absorbance 1.361
c). fully reduced cytochrome: use 900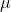 L of 0.300 mg/mL cytochrome c solution mixed with 100
L of 0.300 mg/mL cytochrome c solution mixed with 100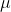 L of 60.0mM ascorbic acid. Since
L of 60.0mM ascorbic acid. Since 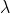 =550nm has an absorbance 0.56
=550nm has an absorbance 0.56
d). Fully oxdized cytochrome c: use 900 L of 0.300 mg/mL cytochrome c solution mixed with 100
L of 0.300 mg/mL cytochrome c solution mixed with 100 L of 60.0mM ferricyanide. Since
L of 60.0mM ferricyanide. Since 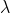 =530nm has an absorbance 0.27
=530nm has an absorbance 0.27
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


