Question: Calculate the total work done for the path (a b c) of (3 mole) of ideal gas a b P v Cv=20.786 J. molek1 Tb=
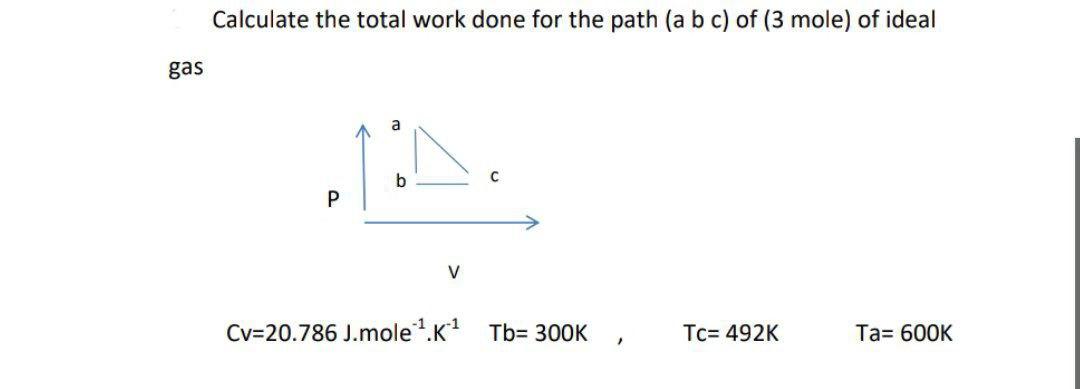
Calculate the total work done for the path (a b c) of (3 mole) of ideal gas a b P v Cv=20.786 J. molek1 Tb= 300K Tc= 492K Ta= 600K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


