Question: can i have solution for 5 and 6 please A 22.69mL sample of liquid bleach (contains NaOCl ) was diluted to 1000mL in a volumetric
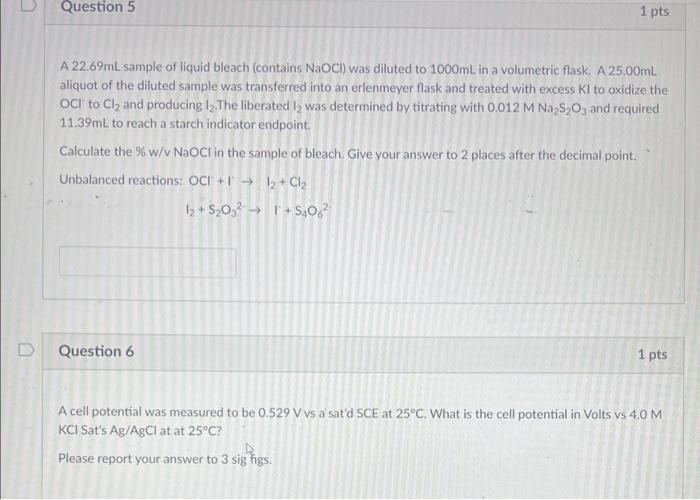
A 22.69mL sample of liquid bleach (contains NaOCl ) was diluted to 1000mL in a volumetric flask. A 25.00mL aliquot of the diluted sample was transferred into an erlenmeyer flask and treated with excess KI to oxidize the OCl to Cl2 and producing I2. The liberated I2 was determined by titrating with 0.012MNa2S2O3 and required 11.39mL to reach a starch indicator endpoint. Calculate the % w/v NaOCl in the sample of bleach. Give your answer to 2 places after the decimal point. Unbalanced reactions: OCl+II2+Cl2 I2+S2O32I4O62 Question 6 1 pts A cell potential was measured to be 0.529Vvs a sat'd SCE at 25C. What is the cell potential in Volts vs 4.0M KClSatsAg/AgCl at at 25C ? Please report your answer to 3 sig higs. A 22.69mL sample of liquid bleach (contains NaOCl ) was diluted to 1000mL in a volumetric flask. A 25.00mL aliquot of the diluted sample was transferred into an erlenmeyer flask and treated with excess KI to oxidize the OCl to Cl2 and producing I2. The liberated I2 was determined by titrating with 0.012MNa2S2O3 and required 11.39mL to reach a starch indicator endpoint. Calculate the % w/v NaOCl in the sample of bleach. Give your answer to 2 places after the decimal point. Unbalanced reactions: OCl+II2+Cl2 I2+S2O32I4O62 Question 6 1 pts A cell potential was measured to be 0.529Vvs a sat'd SCE at 25C. What is the cell potential in Volts vs 4.0M KClSatsAg/AgCl at at 25C ? Please report your answer to 3 sig higs
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


