Question: can someone answer all 5 questions and show all work please! 1. The gas-phase decomposition of ammonia into hydrogen and nitrogen in the presence of
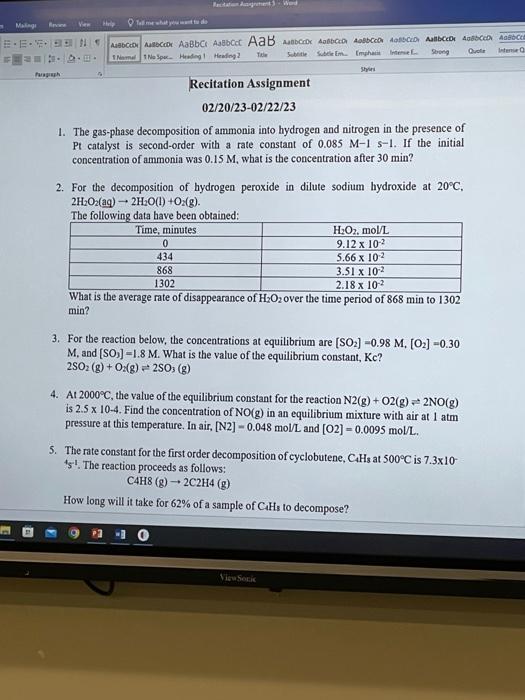
1. The gas-phase decomposition of ammonia into hydrogen and nitrogen in the presence of Pt catalyst is second-order with a rate constant of 0.085M1s1. If the initial concentration of ammonia was 0.15M, what is the concentration after 30min ? 2. For the decomposition of hydrogen peroxide in dilute sodium hydroxide at 20C, 2H2O2(aq)2H2O(l)+O2(g). The followine data have been obtained: What is the average rate of disappearance of H2O2 over the time period of 868min to 1302 min ? 3. For the reaction below, the concentrations at equilibrium are [SO2]=0.98M,[O2]=0.30 M, and [SO3]=1.8M. What is the value of the equilibrium constant, Kc ? 2SO2(g)+O2(g)2SO3(g) 4. At 2000C, the value of the equilibrium constant for the reaction N2(g)+O2(g)2NO(g) is 2.5104. Find the concentration of NO(g) in an equilibrium mixture with air at 1atm pressure at this temperature. In air, [N2]=0.048mol/L and [O2]=0.0095mol/L. 5. The rate constant for the first order decomposition of cyclobutene, C4H3 at 500C is 7.310 41. The reaction proceeds as follows: C4H8(g)2C2H4(g)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


