Question: Can someone answer these questions with the steps please? Q14.1. Nitric oxide gas (NO) reacts with chlorine gas according to the equation NO+1/2Cl2NOCl The following


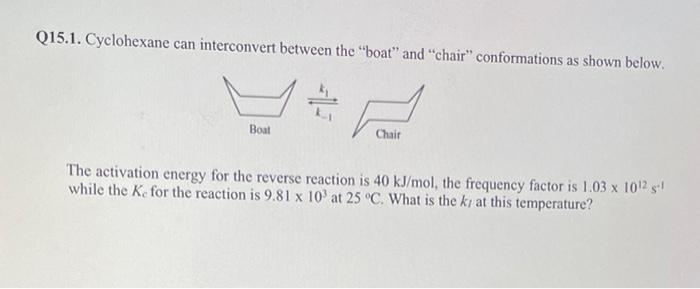


Q14.1. Nitric oxide gas (NO) reacts with chlorine gas according to the equation NO+1/2Cl2NOCl The following initial rates of reaction have been meseurad fonit. reagent concentrations. What is the rate law for the reaction? Q14.2. The activation energy, Ea, for the denaturation of a specific protein is 400kJ/mol. At what temperature will the rate of denaturation be 65 percent greater than the rate at 20C ? 215.1. Cyclohexane can interconvert between the "boat" and "chair" conformations as shown below. The activation energy for the reverse reaction is 40kJ/mol, the frequency factor is 1.031012s1 while the Kc for the reaction is 9.81103 at 25C. What is the kr at this temperature? Q15.2. For the reaction: PCl5(g)PCl3(g)+Cl2(g),Kc=0.80 at a certain temperature. A mixture of 1.50molPCls(g),2.00molPCl3(g) and 0.500molCl2(g) was placed in a 2.00-Liter closed cylinder. Determine the concentration of all species when the system reaches equilibrium. Q16.1. An aqueous solution of barium hydroxide, Ba(OH)2, is found to have a pH of 11.50 at 25C. Show how to determine the concentration of barium ion [Ba2+] ? Q16.2. The ionization constants for sulfurous acid, H2SO3, at 25C are Kal=1.3102 and K22=6.310 -8. Calculate the equilibrium concentrations of H2SO3,H+,HSO3, and SO32 in a 0.15M aqueous solution of sulfurous acid at 25C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


