Question: Can someone help me solve these questions I will be grateful, General Chemistry (c) 9(CuSO43H2O) Element Symbol Number of Atoms Cu S O H How
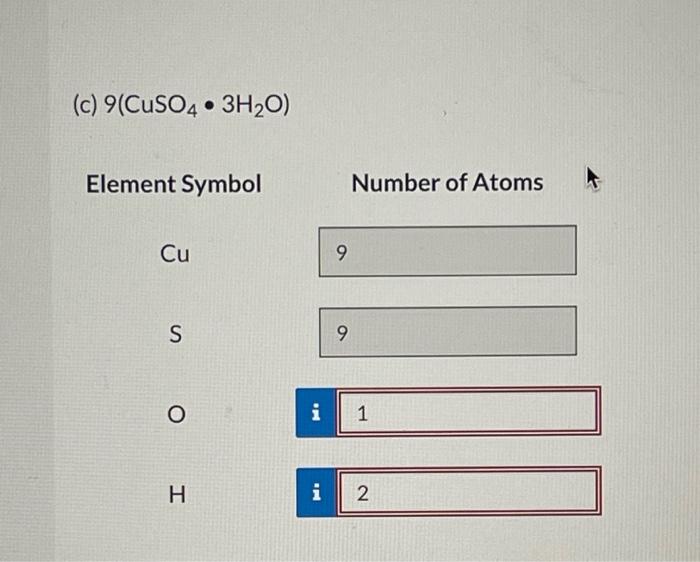
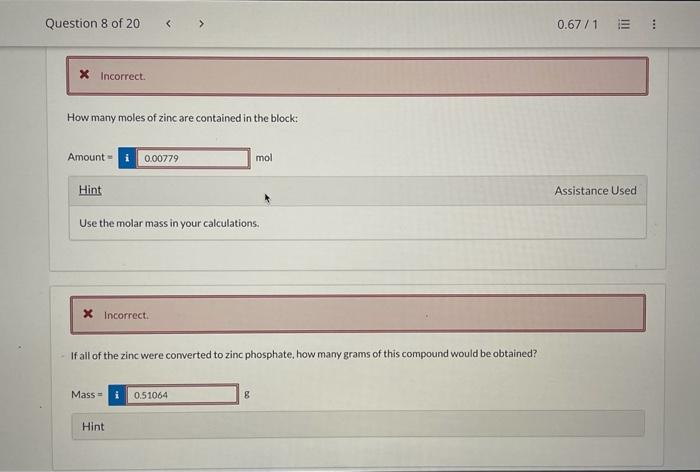
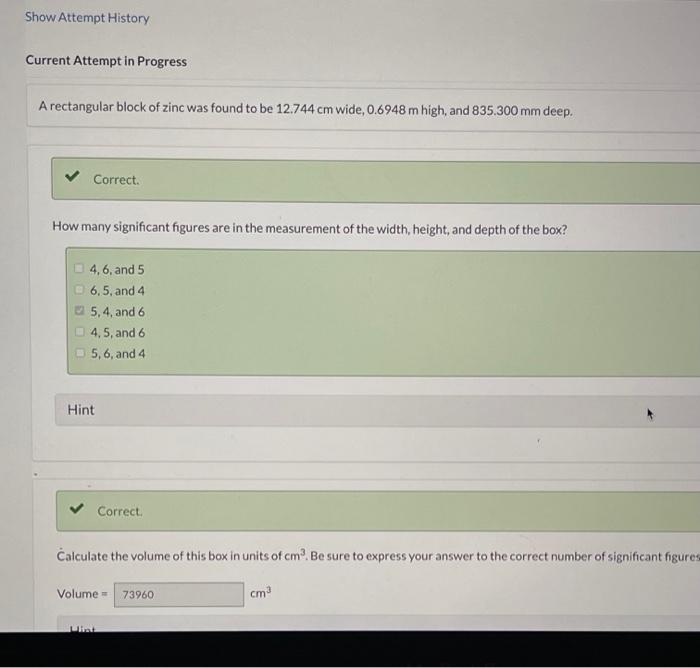
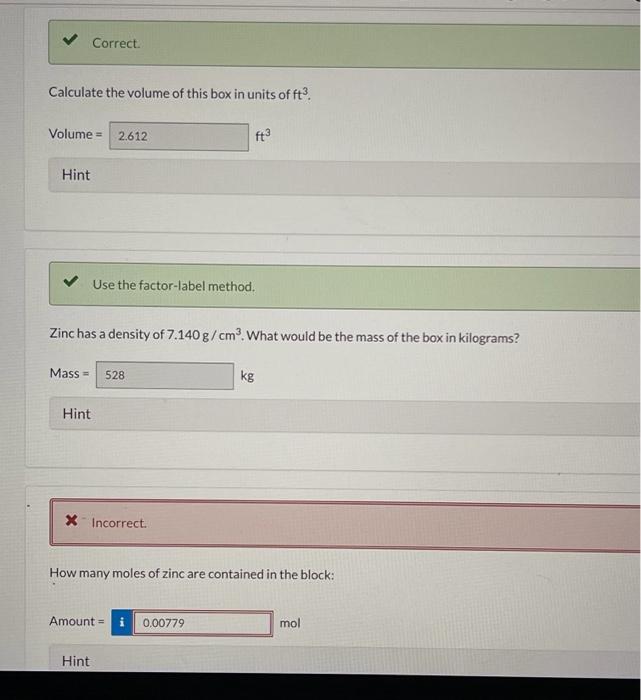
(c) 9(CuSO43H2O) Element Symbol Number of Atoms Cu S O H How many moles of zinc are contained in the block: Amount=1mol Hint Use the molar mass in your calculations. If all of the zinc were converted to zinc phosphate, how many grams of this compound would be obtained? A rectangular block of zinc was found to be 12.744cm wide, 0.6948m high, and 835.300mm deep. Correct. How many significant figures are in the measurement of the width, height, and depth of the box? 4,6 , and 5 6,5, and 4 5. 4 , and 6 4,5 , and 6 5,6, and 4 Hint Correct. Calculate the volume of this box in units of cm3. Be sure to express your answer to the correct number of significant figure Volume= Calculate the volume of this box in units of ft3. Volume = Hint Use the factor-label method. Zinc has a density of 7.140g/cm3. What would be the mass of the box in kilograms? Mass = Hint x incorrect. How many moles of zinc are contained in the block
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


