Question: can someone help me solve this post lab questions please Date: 01/18/22 Your Unknown No.: UV-vis ABSORBANCE DATA @ 590 nm Volume (mL) of 12.5
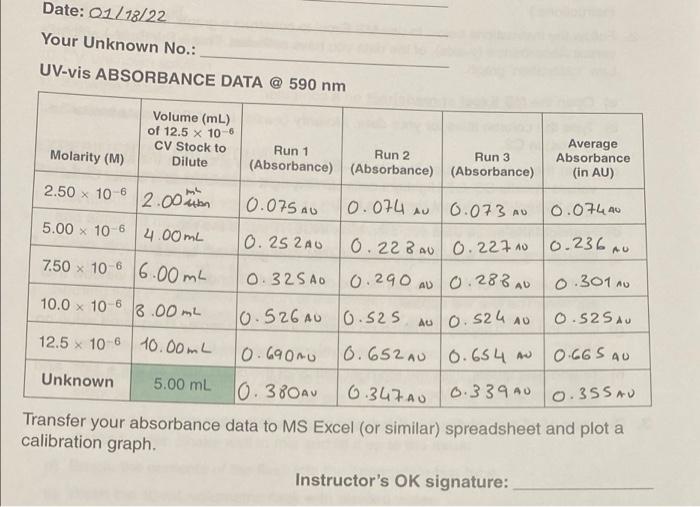

Date: 01/18/22 Your Unknown No.: UV-vis ABSORBANCE DATA @ 590 nm Volume (mL) of 12.5 x 10-6 CV Stock to Dilute Molarity (M) Run 1 Run 2 Run 3 (Absorbance) (Absorbance) (Absorbance) Average Absorbance (in AU) x 10-6 AU X AU 7.50 x 10-6 6.00 mL X AU 2.50 2.00mm 0.075 AU 10.074 su 0.073 AU 0.074A0 5.00 x 10-6 4.00ml O. 252AO 0.22B 0.22710 0.236.0 0.32 SAD 0.290 0.2880 0.301 10.0 x 10 6 13.00mL 10.526A0 0.525 Au0.524 no 0.52 SAU 12.5 x 10-6 10.00mL 10.690nu 10.652 AU 0.654 A 0.665 AO Unknown 5.00 mL 10.30AU 6.347A0 0.339.00.355 AU Transfer your absorbance data to MS Excel (or similar) spreadsheet and plot a calibration graph Instructor's OK signature: 29 Beneath the Things We See POST-LAB QUESTIONS 1. Your data table on the spreadsheet must have appropriate column titles. Fully label your calibration graph on MS Excel,Include your graph with this report along with the data table 2. Given that the cuvette used in this experiment has a path length of 1.0 cm, calculate the molar absorptivity (e) of CV solution using the Beer-Lambert law. A = ebC, at any absorbance (average) along with its corresponding standard molarity. Comment on how your calculated answer compares to the slope (eb) of the calibration graph (in AU/mol.L) when y-intercept is zero. The molor absobtion at any obsorbana considering y interapt is o" will be given as such A = Ebc let, A= 3. Use the equation of the calibration graph to determine the molarity (M) in the unknown CV solution Show your work. 4. If the unknown CV solution you analyzed was made by diluting a 0.50-ml sample of the original to 10.00 ml, calculate the molarity of the original CV solution -000- END OF LAB REPORT
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


