Question: Can someone please answer question 4 and 5 Consider the following reaction, equilibrium concentrations, and equilibrium constant at a particular temperature. Determine the equilibrium pressure
Can someone please answer question 4 and 5
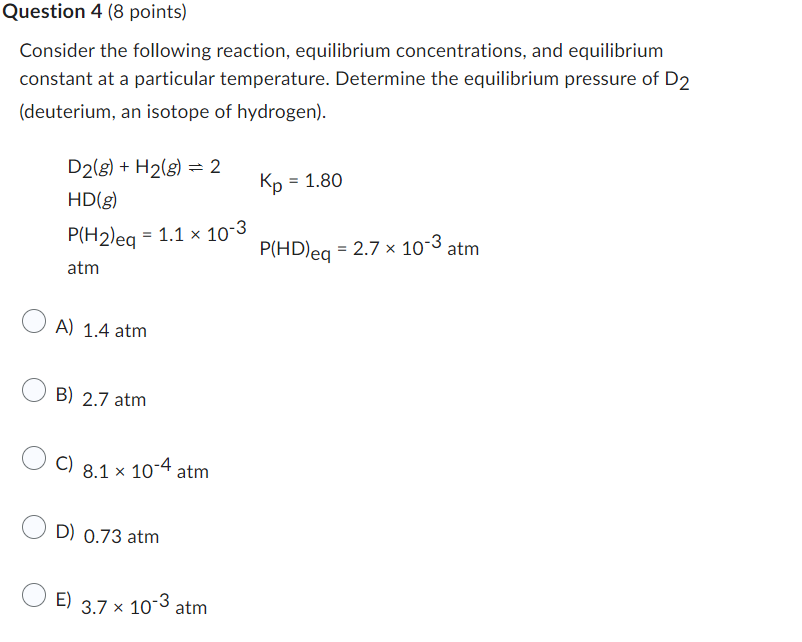

Consider the following reaction, equilibrium concentrations, and equilibrium constant at a particular temperature. Determine the equilibrium pressure of D2 (deuterium, an isotope of hydrogen). D2(g)+H2(g)=2HD(g)P(H2)eq=1.1103atmKp=1.80P(HD)eq=2.7103atm A) 1.4atm B) 2.7atm C) 8.1104atm D) 0.73atm E) 3.7103atm Use the standard reaction enthalpies given below to determine Hrxn for the following reaction: P4(g)+10Cl2(g)4Hrxn=?PCl5(s) Given: PCl5(s)PCl3(g)+Cl2(g)P4(g)+6Cl2(g)4PCl3(g)Hrxn=+157kJHrxn=1207kJ A) 2100.kJ B) 1050.kJ C) 1786kJ D) 1835kJ E) 1364kJ
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


