Question: can you help me with question 2a, 2b abd question 3 a and 3 b a. Derive the formula for the change in concentration on
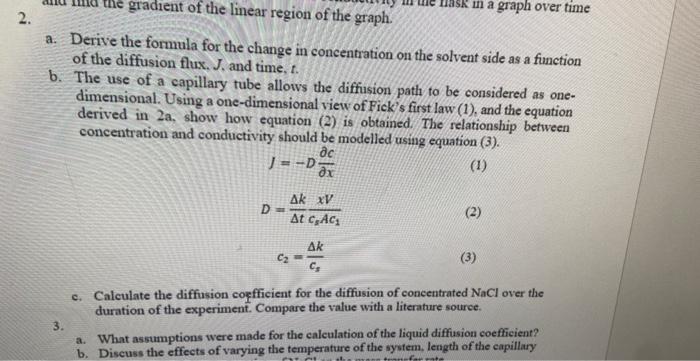
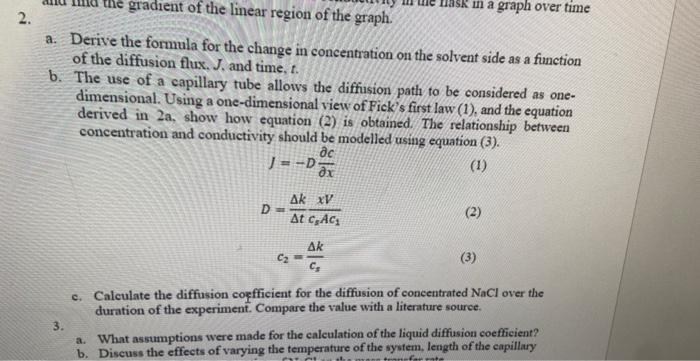
a. Derive the formula for the change in concentration on the solvent side as a function of the diffusion flux. J, and time, t. b. The use of a capillary tube allows the diffusion path to be considered as onedimensional. Using a one-dimensional view of Fick's first law (1), and the equation derived in 2a, show how equation (2) is obtained. The relationship between concentration and conductivity should be modelled using equation (3). J=DxcD=tkcsAc1xVc2=csk c. Calculate the diffusion coefficient for the diffusion of concentrated NaCl over the duration of the experiment. Compare the value with a literature source. 3. a. What assumptions were made for the calculation of the liquid diffusion coefficient? b. Discuss the effects of varying the temperature of the system, length of the capillary a. Derive the formula for the change in concentration on the solvent side as a function of the diffusion flux. J, and time, t. b. The use of a capillary tube allows the diffusion path to be considered as onedimensional. Using a one-dimensional view of Fick's first law (1), and the equation derived in 2a, show how equation (2) is obtained. The relationship between concentration and conductivity should be modelled using equation (3). J=DxcD=tkcsAc1xVc2=csk c. Calculate the diffusion coefficient for the diffusion of concentrated NaCl over the duration of the experiment. Compare the value with a literature source. 3. a. What assumptions were made for the calculation of the liquid diffusion coefficient? b. Discuss the effects of varying the temperature of the system, length of the capillary
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


