Question: Can you help solve this ? Problem 4: Determine the molarity and normality of 15% sulfuric acid (H2SO4):a. By mass b. By volume Assume the
Can you help solve this ?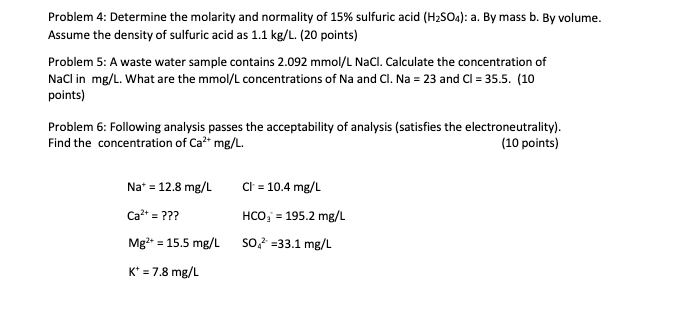
Problem 4: Determine the molarity and normality of 15% sulfuric acid (H2SO4):a. By mass b. By volume Assume the density of sulfuric acid as 1.1kg/L. (20 points) Problem 5: A waste water sample contains 2.092mmol/LNaCl. Calculate the concentration of NaCl in mg/L. What are the mmol/L concentrations of Na and Cl. Na=23 and Cl=35.5. (10 points) Problem 6: Following analysis passes the acceptability of analysis (satisfies the electroneutrality). Find the concentration of Ca2+mg/L. (10 points) Na+=12.8mg/LCa2+=???Mg2+=15.5mg/LK+=7.8mg/LCl+=10.4mg/LHCO3=195.2mg/LSO42+=33.1mg/L
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


