Question: Can you write the solutions step by step? Q1. Evaluate the difference (TU)P(TU)V for the (a) ideal and (b) van der Waals gases, and for
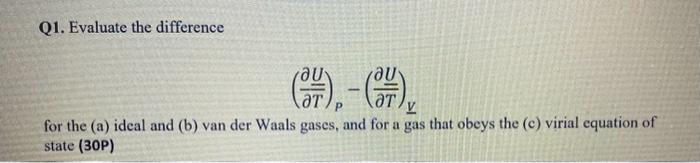
Q1. Evaluate the difference (TU)P(TU)V for the (a) ideal and (b) van der Waals gases, and for a gas that obeys the (c) virial equation of state (30P)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


