Question: cathode. The electrolyte circulated through the battery is NaOH(aq) as depicted by the following figure. a) Write down the half-reactions occurring at anode and cathode,
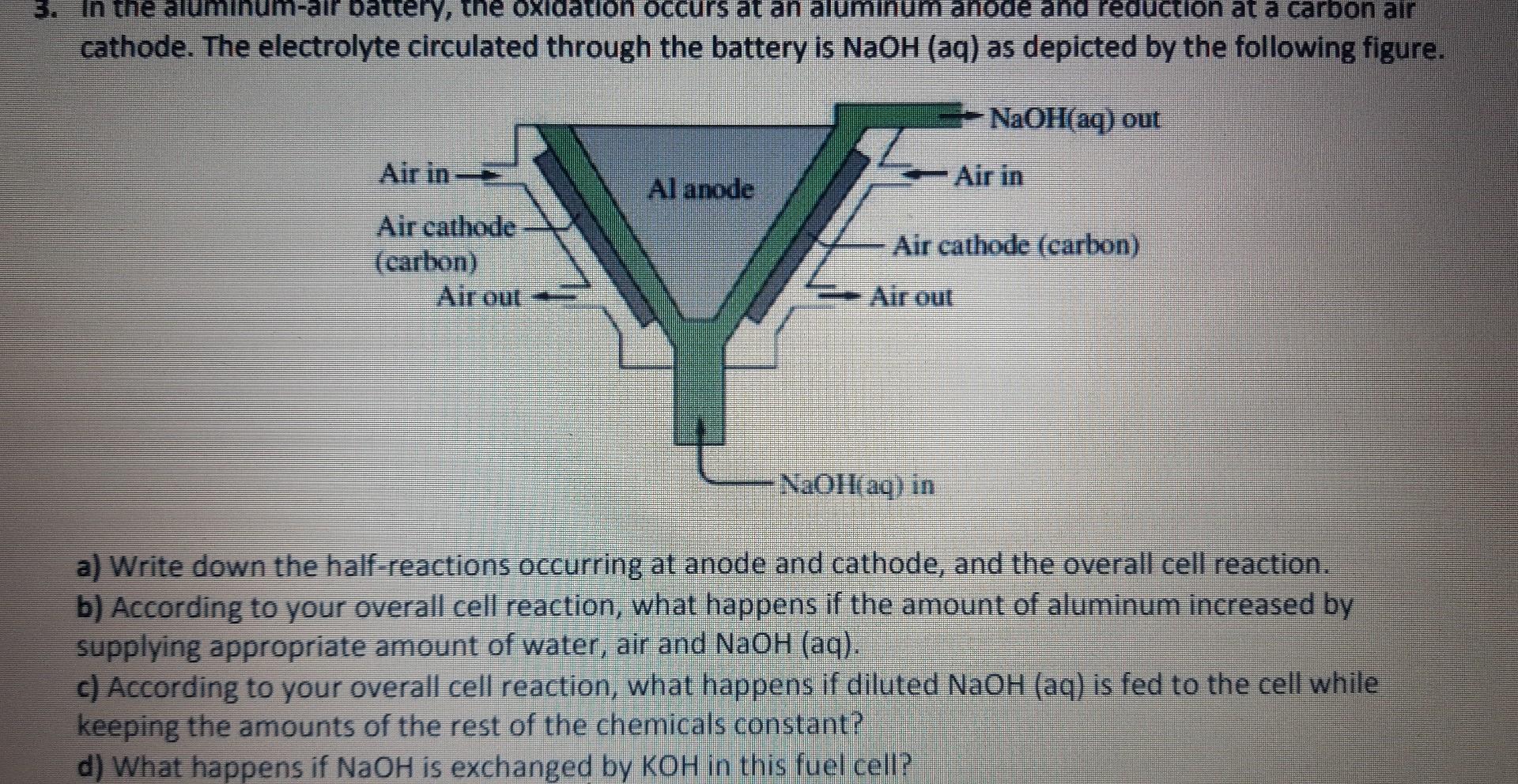
cathode. The electrolyte circulated through the battery is NaOH(aq) as depicted by the following figure. a) Write down the half-reactions occurring at anode and cathode, and the overall cell reaction. b) According to your overall cell reaction, what happens if the amount of aluminum increased by supplying appropriate amount of water, air and NaOH(aq). c) According to your overall cell reaction, what happens if diluted NaOH(aq) is fed to the cell while keeping the amounts of the rest of the chemicals constant? d) What happens if NaOH is exchanged by KOH in this fuel cell
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


