Question: Changes to temperature: Endothermic reaction: Exothermic reaction: 8. reactants + heat products reactants products + heat The reaction: 2SO2(g) + O2(g) 2SO3(g) 160. AH
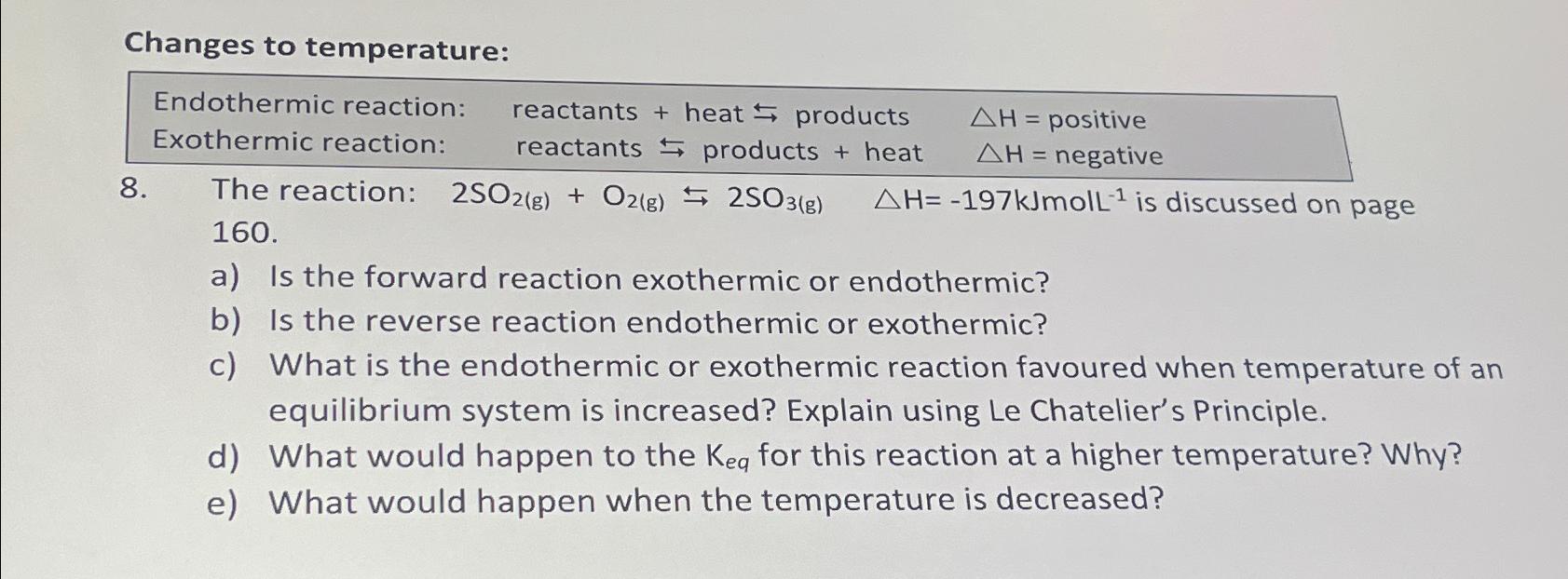
Changes to temperature: Endothermic reaction: Exothermic reaction: 8. reactants + heat products reactants products + heat The reaction: 2SO2(g) + O2(g) 2SO3(g) 160. AH = positive = negative AH=-197kJmoll- is discussed on page a) Is the forward reaction exothermic or endothermic? b) Is the reverse reaction endothermic or exothermic? c) What is the endothermic or exothermic reaction favoured when temperature of an equilibrium system is increased? Explain using Le Chatelier's Principle. d) What would happen to the Keq for this reaction at a higher temperature? Why? e) What would happen when the temperature is decreased?
Step by Step Solution
There are 3 Steps involved in it
Based on the information provided a Exothermic The given equation shows a negative H 197 kJmol which ... View full answer

Get step-by-step solutions from verified subject matter experts


