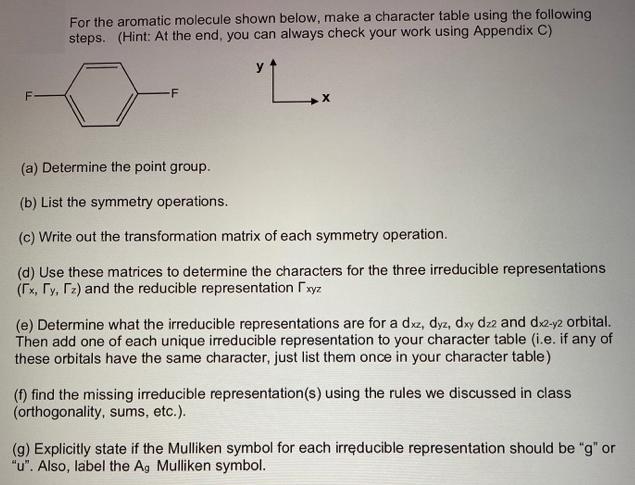
Question: For the aromatic molecule shown below, make a character table using the following steps. (Hint: At the end, you can always check your work
For the aromatic molecule shown below, make a character table using the following steps. (Hint: At the end, you can always check your work using Appendix C) "L. -F (a) Determine the point group. (b) List the symmetry operations. (c) Write out the transformation matrix of each symmetry operation. (d) Use these matrices to determine the characters for the three irreducible representations (Tx, Ty, r2) and the reducible representation xyz (e) Determine what the irreducible representations are for a dxz, dyz, dxy dz2 and dx2-y2 orbital. Then add one of each unique ireducible representation to your character table (i.e. if any of these orbitals have the same character, just list them once in your character table) (f) find the missing irreducible representation(s) using the rules we discussed in class (orthogonality, sums, etc.). (g) Explicitly state if the Mulliken symbol for each irreducible representation should be "g" or "u". Also, label the Ag Mulliken symbol.
Step by Step Solution
3.60 Rating (168 Votes )
There are 3 Steps involved in it
a The point group of the aromatic compound is D 2h b The symmetry operations associated with t... View full answer

Get step-by-step solutions from verified subject matter experts