Question: Ci (aq) + C10 (aq) + 2H+ (aq) -- C12(9) + H200) What effect will increasing (H) at constant temperature have on the reaction represented
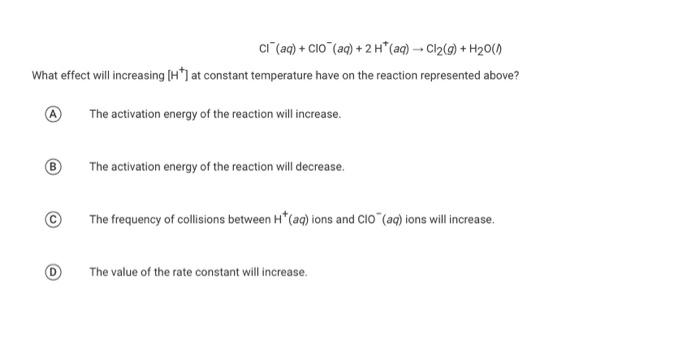
Ci (aq) + C10 (aq) + 2H+ (aq) -- C12(9) + H200) What effect will increasing (H) at constant temperature have on the reaction represented above? The activation energy of the reaction will increase. ( The activation energy of the reaction will decrease The frequency of collisions between H*(aq) ions and Clo" (aq) ions will increase The value of the rate constant will increase
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


