Question: Codeine is a weak organic base with a Kb = 1.6 x 10-6 C18H21 NO3(aq) + H20(1) = C18H24NO3H+ (aq) + OH-(aq) a. What is
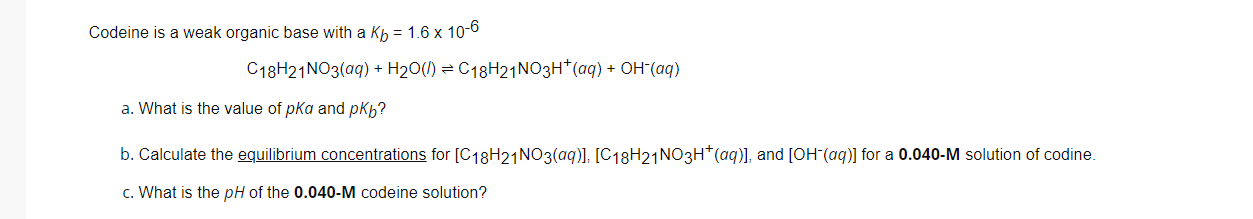
Codeine is a weak organic base with a Kb = 1.6 x 10-6 C18H21 NO3(aq) + H20(1) = C18H24NO3H+ (aq) + OH-(aq) a. What is the value of pka and pkb? b. Calculate the equilibrium concentrations for [C18H24NO3(aq)], [C18H21NO3H+ (aq)], and [OH-(aq)] for a 0.040-M solution of codine. c. What is the pH of the 0.040-M codeine solution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


