Question: Complete a reaction table (in millimoles) for the net reaction resulting when 36.6mL of 0.0660MCu(NO3)2 and 32.1mL of 0.130MKOH are mixed. Enter the net ionic
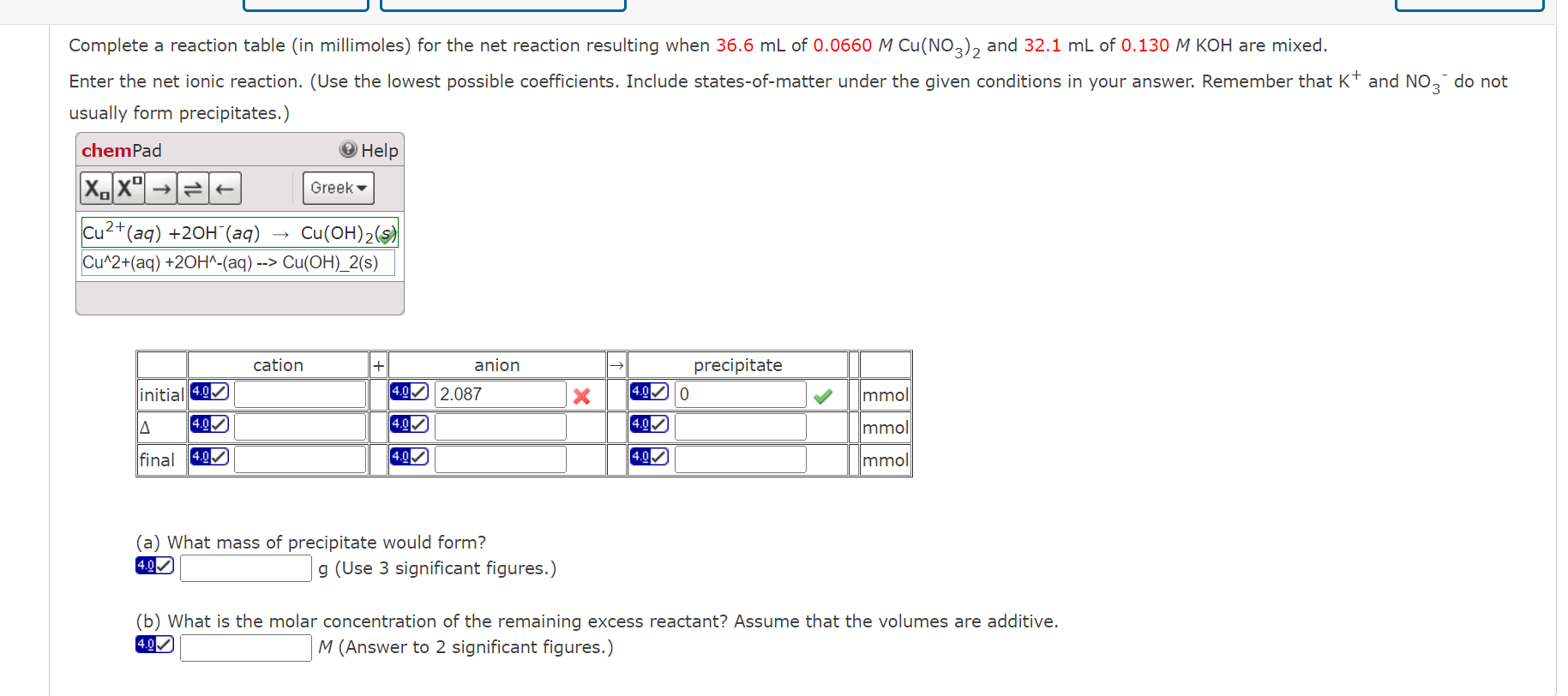
Complete a reaction table (in millimoles) for the net reaction resulting when 36.6mL of 0.0660MCu(NO3)2 and 32.1mL of 0.130MKOH are mixed. Enter the net ionic reaction. (Use the lowest possible coefficients. Include states-of-matter under the given conditions in your answer. Remember that K+and NO3do not usually form precipitates.) (a) What mass of precipitate would form? g.0.0 (Use 3 significant figures.) (b) What is the molar concentration of the remaining excess reactant? Assume that the volumes are additive. 4.gD M (Answer to 2 significant figures.)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


