Question: Compounds A and B react to produce compounds C and D according to the following equation. A+BC+D The following data were collected from several runs
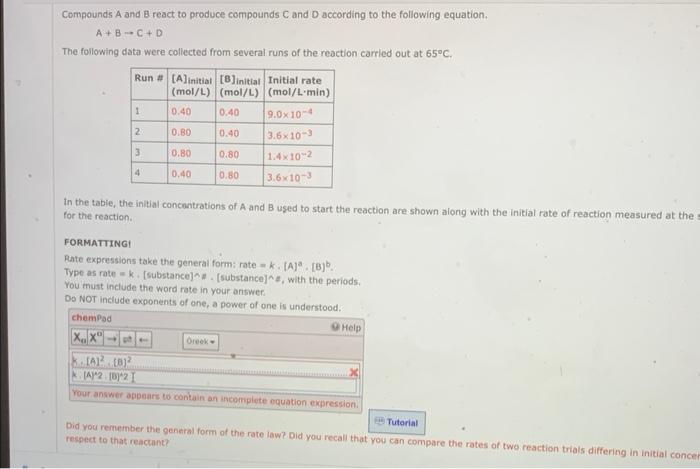
Compounds A and B react to produce compounds C and D according to the following equation. A+BC+D The following data were collected from several runs of the reaction carried out at 65C. In the tabie, the initial concantrations of A and B used to start the reaction are shown along with the initial rate of reaction measured at the for the reaction. FORMATTINGI Rate expressions take the general form: rate =k. [A]a,[B]b. Type as rate =k. [ substance] ]. [substance ]&, with the periods. You must include the word rate in your answer. Do NOT include exponents of one, a power of one is undentinas. Did you remember the general form of the rate law? Did you recall that you can compare the rates of two reaction trials differing in initial conce respect to that reactant
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


