Question: Consider a fluidized CSTR , where the irreversible, elementary reaction A + B 3 C is performed, in an isothermal, isobaric, ideal, gas phase reaction.
Consider a fluidized CSTR where the irreversible, elementary reaction is
performed, in an isothermal, isobaric, ideal, gas phase reaction. The fresh feed contains
equimolar amounts of reactants. Determine how much catalyst is needed to reach a
conversion of
Given: atm,mol catalyst
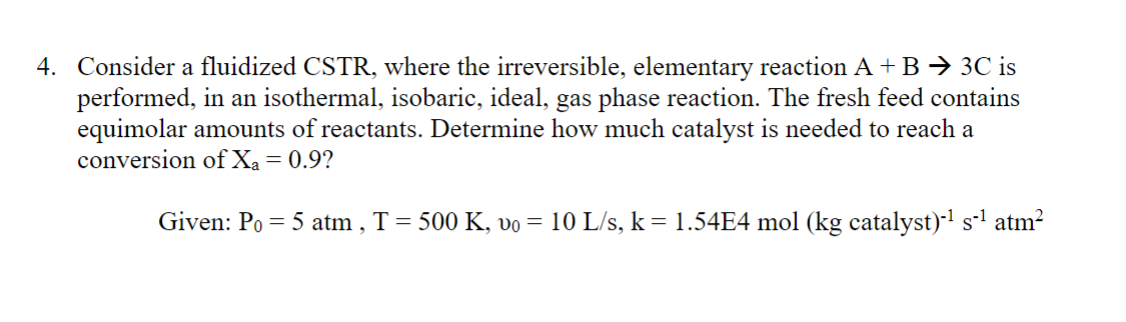
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


