Question: Consider a rocket engine using a kerosene-type propellant similar to RP-1 as fuel and oxygen, O2, as an oxidizer. The fuel and oxidizer enter the
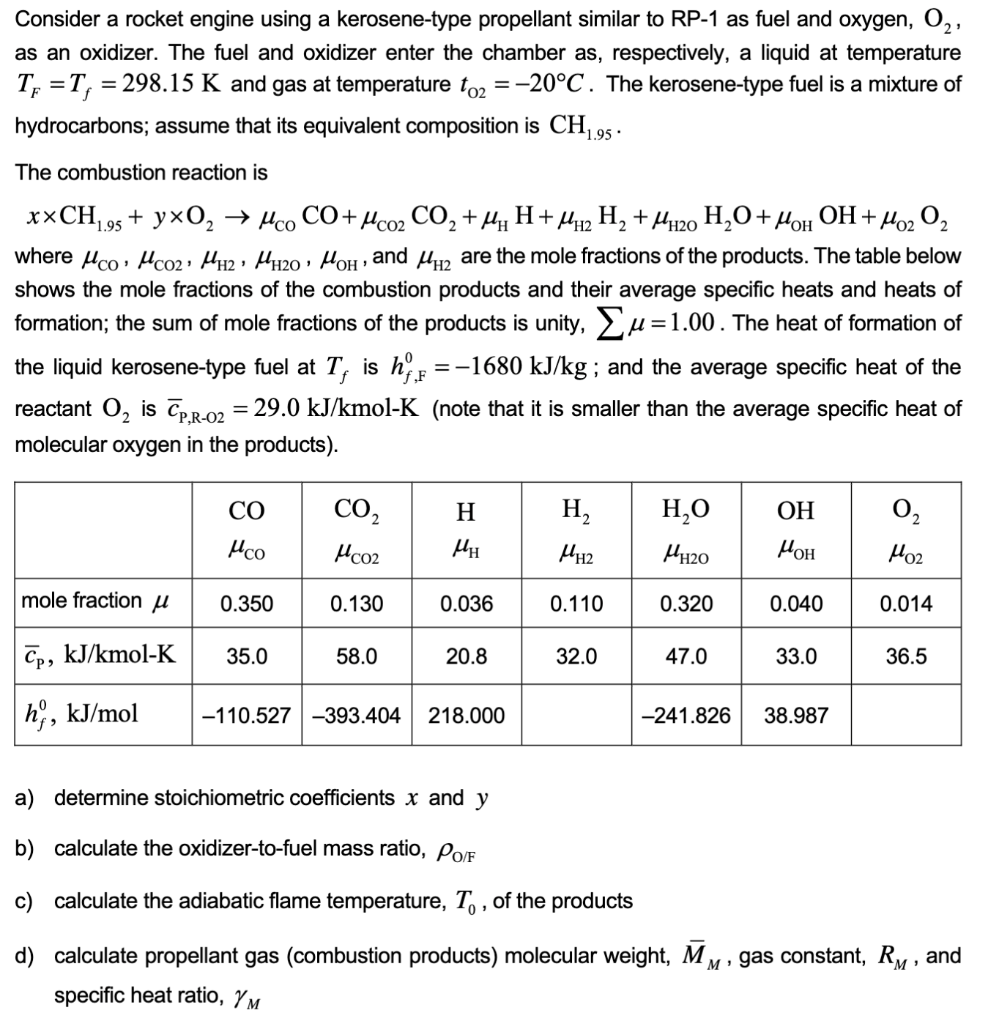
Consider a rocket engine using a kerosene-type propellant similar to RP-1 as fuel and oxygen, O2, as an oxidizer. The fuel and oxidizer enter the chamber as, respectively, a liquid at temperature TF=Tf=298.15K and gas at temperature tO2=20C. The kerosene-type fuel is a mixture of hydrocarbons; assume that its equivalent composition is CH1.95. The combustion reaction is xCH1.95+yO2COCO+CO2CO2+HH+H2H2+H2OH2O+OHOH+O2O2 where CO,CO2,H2,H2O,OH, and H2 are the mole fractions of the products. The table below shows the mole fractions of the combustion products and their average specific heats and heats of formation; the sum of mole fractions of the products is unity, =1.00. The heat of formation of the liquid kerosene-type fuel at Tf is hf,F0=1680kJ/kg; and the average specific heat of the reactant O2 is cP,R02=29.0kJ/kmolK (note that it is smaller than the average specific heat of molecular oxygen in the products). a) determine stoichiometric coefficients x and y b) calculate the oxidizer-to-fuel mass ratio, O/F c) calculate the adiabatic flame temperature, T0, of the products d) calculate propellant gas (combustion products) molecular weight, MM, gas constant, RM, and specific heat ratio, M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


